Indandione compounds and its preparing process and application
A technology of indanedione and indanedihydroxydiketone, which is applied in the field of indanedione compounds, can solve the problems of low reliability, complicated preparation method, high production cost, etc., and achieve high reliability, simple detection method, Effects that are not easy to imitate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 3,3'-Diethyl-3,3'-dihydroxy-2,2'-diindan-1,1'-dione
[0029] The first step: the synthesis of 2,2'-diindan-1,1',3,3'-tetraketone Add 25.0 grams of phthalic anhydride and 20.0 grams of succinic acid into a 500-milliliter four-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, and stir and heat until the solids are completely melted. When all the solids are melted, add 6.0 g of anhydrous potassium acetate, react at 210°C for 2 hours, after cooling, add hot water to boil for 2 hours, and filter. The resulting precipitate was dissolved in methanol, and 50 ml of 2 mol / l sodium methoxide solution was rapidly added dropwise under stirring, and the reaction was refluxed for 0.5 hour, cooled, and filtered. The resulting precipitate was boiled with distilled water for 1 hour and filtered. The filtrate was neutralized to acidity with 1mol / l hydrochloric acid solution, and a purple-red precipitate was precipitated. The purple-red precipitate w...
Embodiment 2
[0036] 3,3'-Dipropyl-3,3'-dihydroxy-2,2'-diindan-1,1'-dione
[0037] The first step: the synthesis of 2,2'-diindan-1,1',3,3'-tetraketone
[0038] The synthesis steps are the same as in Example 1.
[0039] Step 2: Synthesis of Bifunctional Photomagnetic Chromogenic Compounds
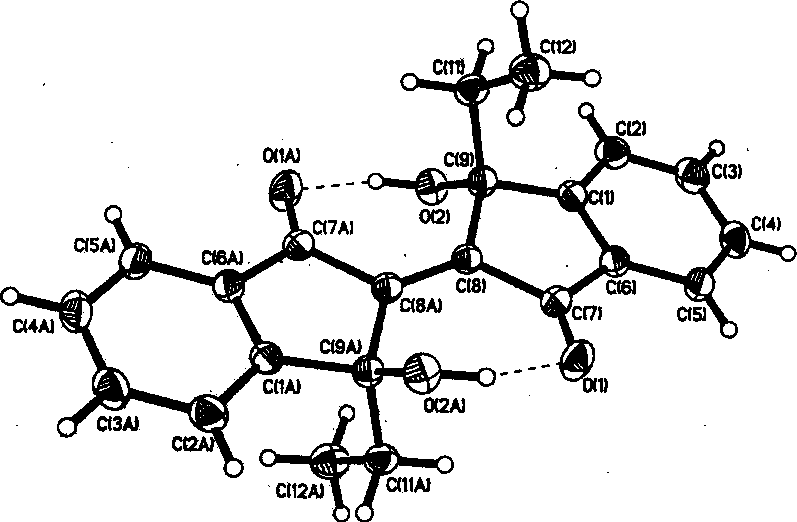
[0040] Add 24.0 g of 2,2'-diindan-1,1',3,3'-tetraketone and 500 ml of benzene After the addition, react at room temperature for 12 hours under the protection of nitrogen, and continue to stir for a period of time. After the reaction is completed, it is hydrolyzed with saturated ammonium chloride solution and left standing, and a yellow precipitate insoluble in the two phases is precipitated. The precipitate was purified by column chromatography and recrystallized with dichloromethane to obtain yellow bifunctional photomagnetic color crystal 3,3'-dipropyl-3,3'-dihydroxy-2,2'-biindane-1 , 1'-diketone 14.3 g, yield: 45.5%. The single crystal structure of the compound is as Figure 4 shown.
[0...
Embodiment 3
[0044] 3,3'-Diphenyl-3,3'-dihydroxy-2,2'-diindan-1,1'-dione
[0045] The first step: the synthesis of 2,2'-diindan-1,1',3,3'-tetraketone
[0046] Mix 20 grams of nindanhydrin with 600 milliliters of sulfuric acid solution, which is prepared from 300 milliliters of concentrated sulfuric acid and 300 milliliters of water, and react for 30 minutes under back distillation. A dark purple solid precipitated out and was filtered. Dissolve the solid with 600 ml of glacial acetic acid, boil for half an hour, cool, pour this solution into a 2000 ml ice-water bath, and precipitate a dark reddish-brown solid. Wash the dark reddish-brown solid with water to remove residual acid solution, dry it, and recrystallize it in toluene to obtain 9.3 g of dark purple-brown needle-like crystals of 2,2'-bisindane-1,1',3,3'-tetraketone , Yield: 57.1%. Melting point: 280-282°C.
[0047] Step 2: Synthesis of Bifunctional Photomagnetic Chromogenic Compounds
[0048] In the ether solution of pheny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com