Method for preparing calcium carbonate with actual forms
A technology of calcium carbonate and morphology, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problems of slow mass transfer rate, wide particle size distribution, poor micro-mixing, etc., to shorten the reaction time, reduce production costs, and narrow particle size distribution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
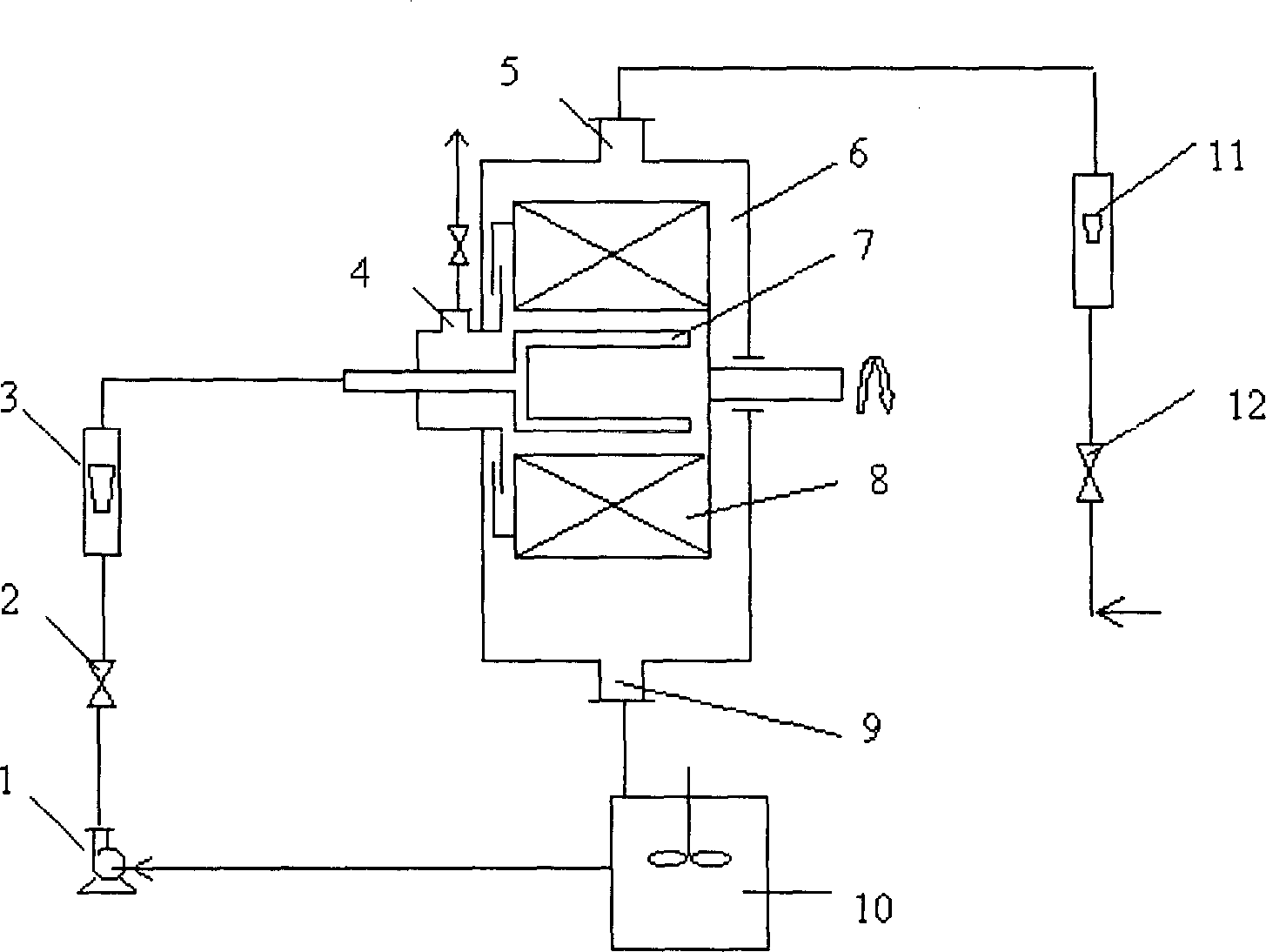
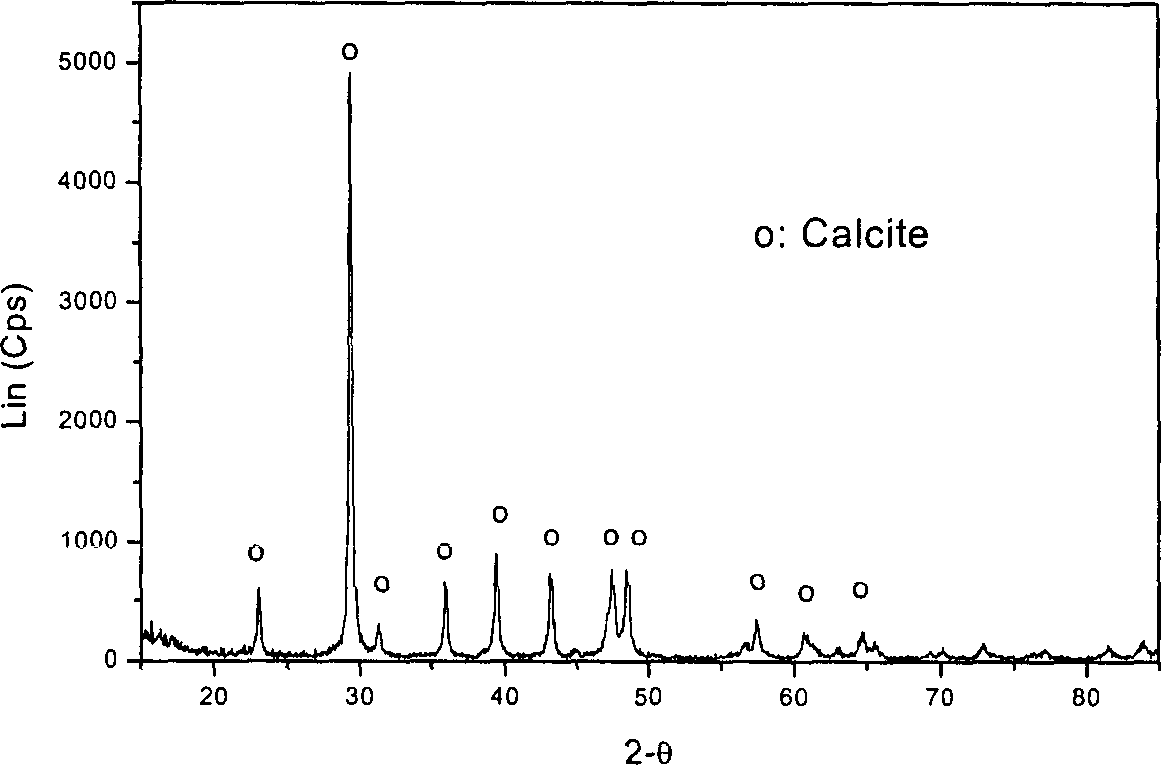
[0047] According to the measurement, take 5kg CaO in the stirred tank, add water with a temperature above 95°C in the quicklime according to the gray-to-water ratio of 1:10 by weight, stir evenly, cool, and then filter the slag with a standard inspection sieve to obtain Ca(OH) 2 Raw material solution, the raw material solution is roughly mixed with water to contain Ca 2+ 0.8mol / L Ca(OH) 2 Suspension. Determination of Ca(OH) by EDTA complexometric titration 2 Ca in suspension 2+ the exact concentration. Adopt technological process of the present invention (as figure 1 ), the prepared Ca(OH) 2 Suspension 3.51 is added to the stirring tank 10 and sent out through the pump 1, and after the liquid flow meter 3 measures it, it is 0.3m 3 The flow of / h enters the porous packing layer 8 through the distributor 7, and the industrial pure CO 2 After the gas is decompressed and measured by the gas flow meter 11, it is measured by 0.3m 3 The flow rate of / h enters the reactor, in...
Embodiment 2
[0051] Except following change, all the other are the same as embodiment 1.
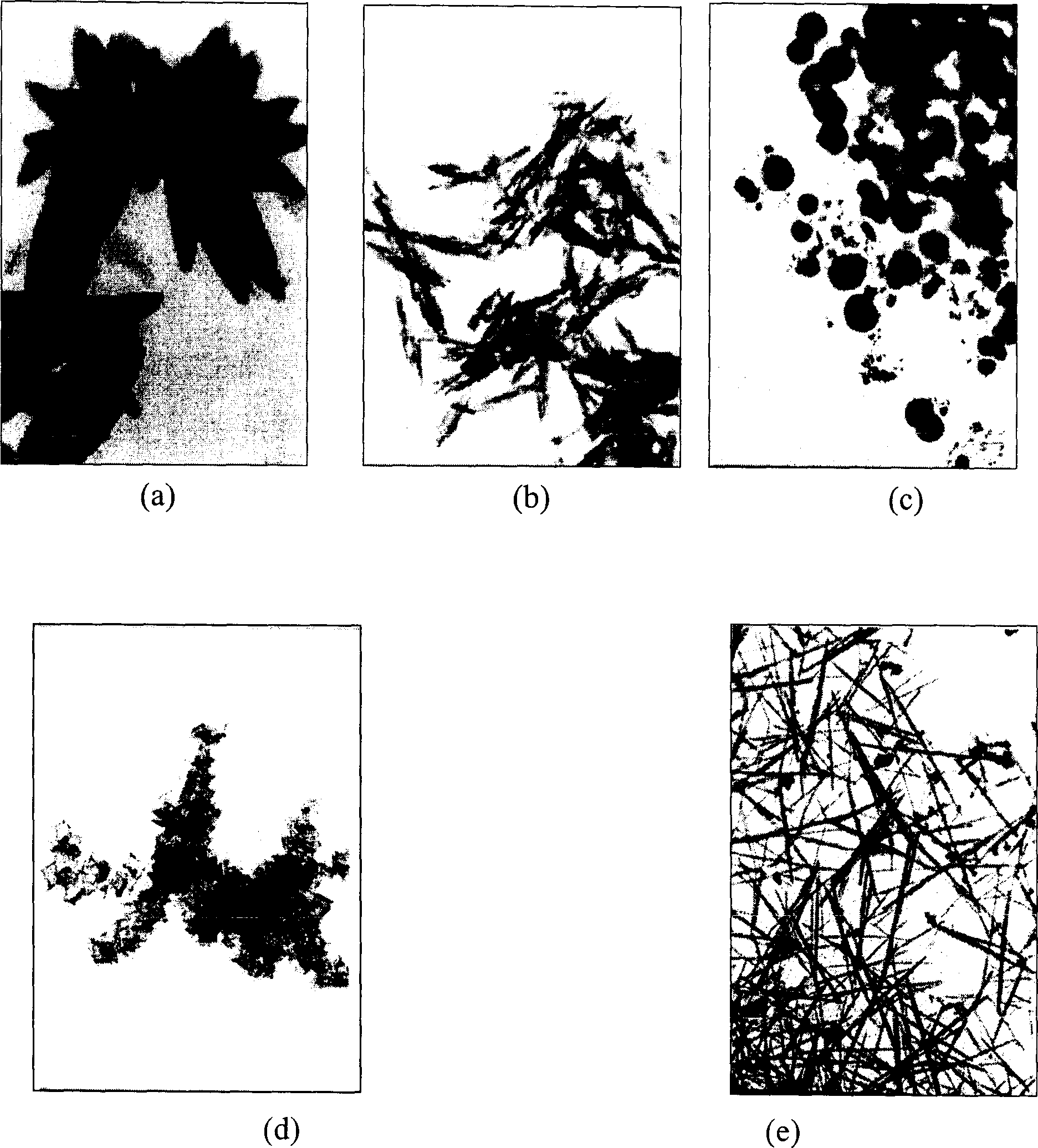
[0052]The temperature of the carbonization reaction is controlled in the range of about 50-60° C., and the dispersibility of the obtained product is better than that of Example 1, and the morphology is more inclined to the spindle shape. Other product characteristics are with embodiment 1.
Embodiment 3
[0054] Except following change, all the other are the same as embodiment 1.
[0055] The gas flow rate is 0.5m 3 / h, the entire carbonization reaction time is shortened, the particle diameter is 1 μm, the short diameter is 0.3 μm, and the morphology is still spindle-shaped.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Short diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com