Polymer micelle medicine-carrying system
A drug-loading system and polymer technology, which can be used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problem of low active targeting, and achieve the effect of improving drug loading and physical stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment is the preparation of the methyl polyethylene glycol-polylactic acid block copolymer that prepares etoposide and the mensuration of molecular weight, CMC
[0029] Materials and Methods
[0030] D, L-lactide: purchased from Sigma, recrystallized three times with anhydrous ethyl acetate at 60°C before use, and at room temperature in P 2 o 5 Dry in medium vacuum for 24 hours. Etoposide, stannous octoate and methyl polyethylene glycol (molecular weight 2000) were purchased from Sigma.
[0031] 1. Synthesis of methyl polyethylene glycol-polylactic acid block copolymer:
[0032] A ring-opening polymerization method is adopted to prepare methyl polyethylene glycol-polylactic acid block copolymers with a mass ratio of 5 / 95, or 20 / 80, or 50 / 50, respectively. A) Weigh 0.5g of methyl polyethylene glycol and 9.5g of lactide to prepare 5 / 95 methyl polyethylene glycol-polylactic acid block copolymer; B) Weigh 2g of methyl polyethylene glycol and lactide Ester 8g pr...
Embodiment 2
[0047] Preparation and particle size determination of etoposide-methylpolyethylene glycol-polylactic acid block copolymer micelles
[0048] 1. Preparation of etoposide-methyl polyethylene glycol-polylactic acid block copolymer micelles
[0049] Weigh 50 mg of etoposide and 100 mg of methyl polyethylene glycol-polylactic acid block copolymer respectively into a flask, add 50 ml of acetonitrile, and stir until dissolved. Under continuous stirring, 25ml of water was slowly added dropwise, stirring was continued for 15 minutes, and 75ml of water was further added dropwise. Slowly evaporate acetonitrile (rotary evaporator) at 60°C under vacuum. After no obvious acetonitrile evaporates, increase the temperature and vacuum, and continue to evaporate to a sample volume of about 80ml. The sample volume is adjusted to 100ml with water. The product is sterilized by filtration with a 0.22mm microporous membrane.
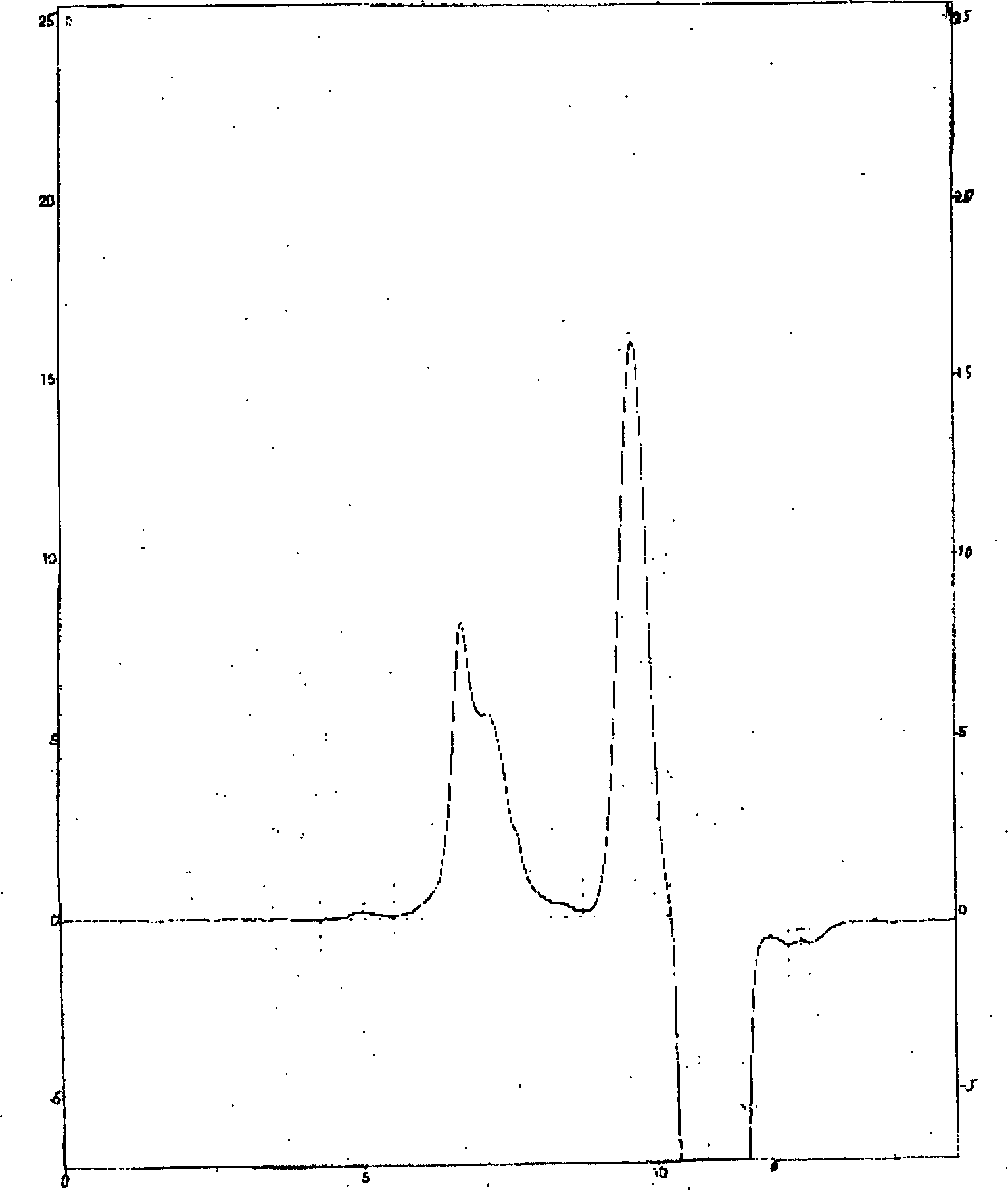
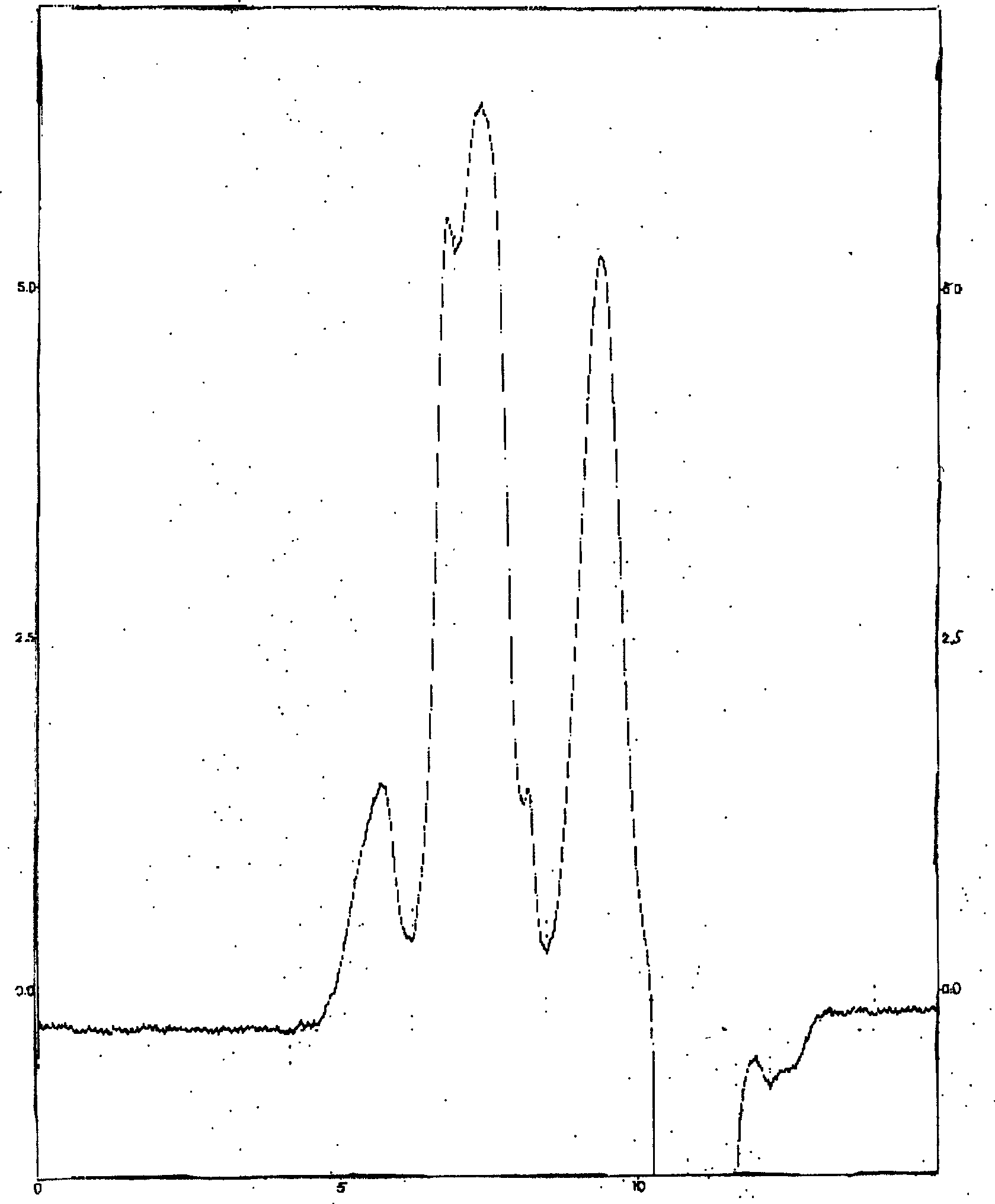
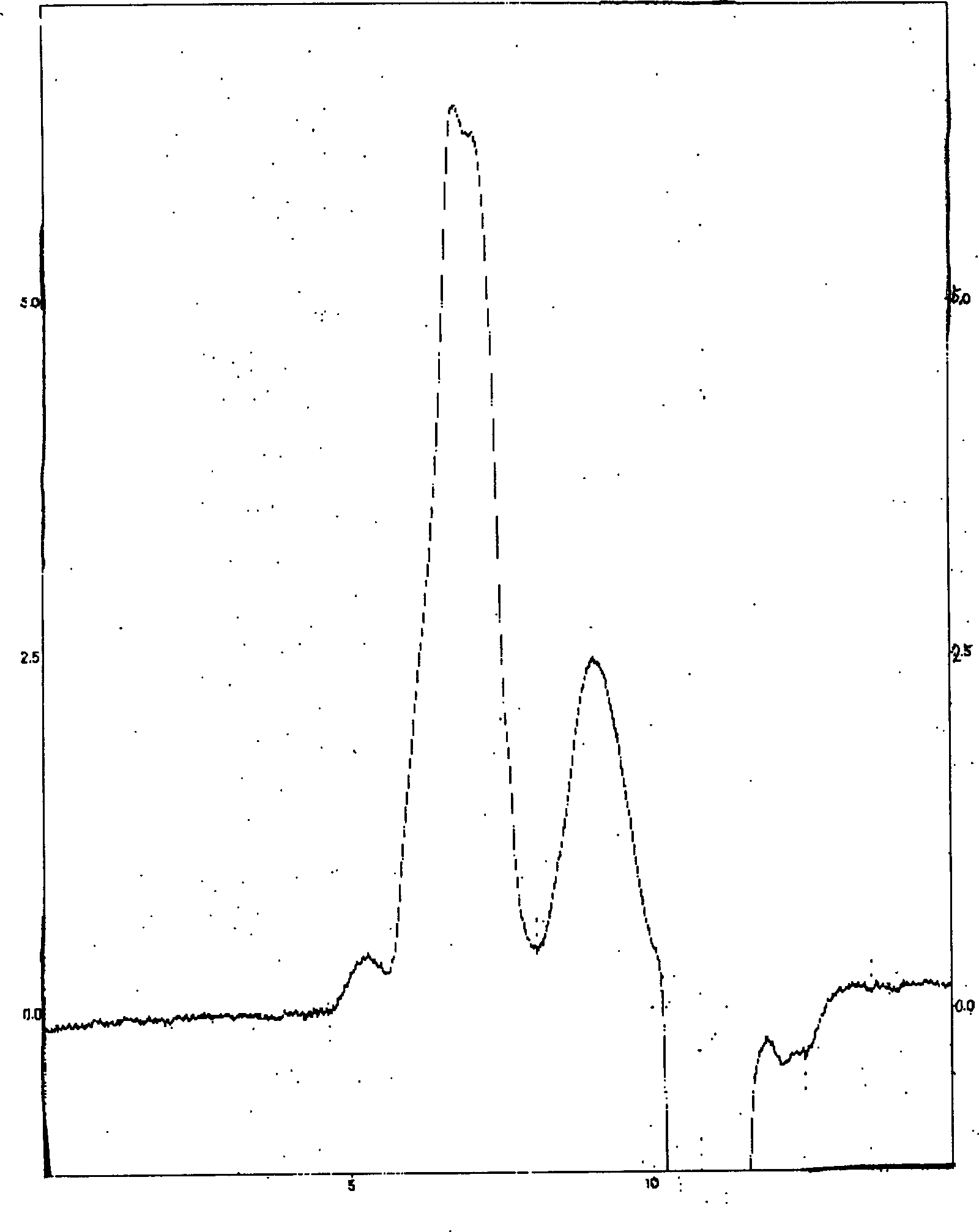
[0050] 2. Particle size determination of etoposide-methyl polyethylene gl...
Embodiment 3
[0053] Preparation of RGD-stearic acid amide Preparation of stearic acid-N-succinimide: Take 0.02 mole of stearic acid and 0.02 mole of N-hydroxysuccinimide, and dissolve them in 20 ml of tetrahydrofuran at 0°C with constant stirring. 0.02 mol of dicyclohexylcarbodiimide was added and stirred overnight at room temperature. The solvent was removed by filtration, and the residue was washed with sodium bicarbonate and recrystallized from methanol. Preparation of dimethyl RGD: 500 mg of RGD (Arg-Gly-Asp tripeptide, Bachem) was dissolved in 20 ml of methanol and kept at 0°C. Add 2ml of thionyl(di)chloride and stir overnight at room temperature. Evaporate the solvent under reduced pressure to obtain dimethyl RGD.
[0054] The preparation of dimethyl RGD-stearic acid amide: get equimolar dimethyl RGD solution (dimethylformamide is solvent) and stearic acid N-succinimide solution (ethylene glycol dimethyl ether is solvent) and mix well, add an appropriate amount of triethylamine to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com