Carboxybutyryl chitosan sulfuric ester and preparation method and use thereof
A technology of carboxybutyryl chitosan sulfate and chitosan sulfate, which is applied in the field of carboxybutyryl chitosan sulfate, can solve the problems of difficult sulfonation reaction and carboxyl dissociation, and achieve easy carboxyl substitution Control, less side effects, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
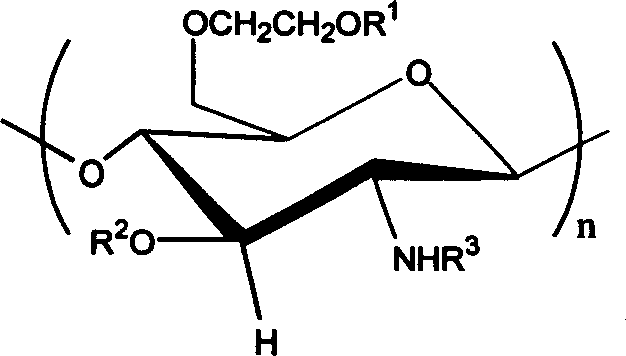
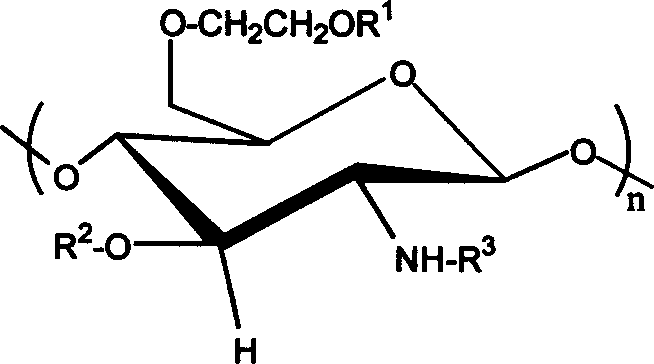
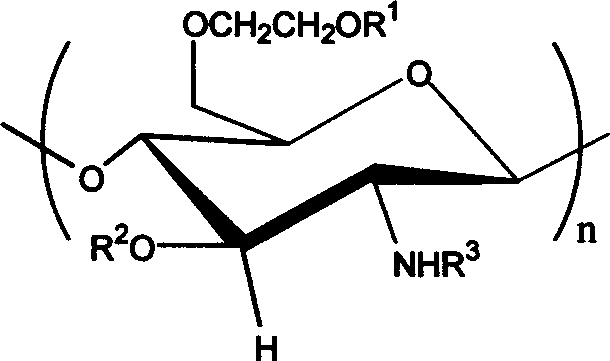
[0023] 4.20g sulfur content is that the chitosan sulfate ester of 16.87% (weight ratio) is dissolved in the NaOH / glycine buffer solution (I=1mol / L) of 9.0 in 20ml pH, stirs at room temperature, divides and adds 0.6g butanediol in two times Acid anhydride (molar ratio of chitosan sulfate sugar unit to succinic anhydride 0.6) was stirred for 24 hours, and the pH was adjusted to 9.0-10.0; the reaction solution was put into a dialysis bag and dialyzed for 3 days, and concentrated to obtain 0.5 g of brown powder, which was N- Carboxybutyryl chitosan sulfate. The substitution degree of elemental analysis is 0.77mol / unit, gel chromatography proves that the difference between molecular weight and chitosan sulfate is very small, there is no degradation phenomenon in the reaction process, and the corresponding group characteristic absorption appears in infrared analysis, carboxyl (1560cm -1 and 1414cm -1 ) and amidocarbonyl (1640cm -1 ) without ester carbonyl absorption (1720cm -1 ),...
Embodiment 2
[0025] As in Example 1, the amount of succinic anhydride is 0.2g (the molar ratio of chitosan sulfate ester sugar unit to succinic anhydride is 0.2), and the elemental analysis degree of substitution is 0.18mol / unit. The activated partial thromboplastin time and thrombin time reached 1.1 times and 2 times of the corresponding chitosan sulfate respectively.
Embodiment 3
[0027] As in Example 1, the amount of succinic anhydride is 0.8g (the molar ratio of chitosan sulfate sugar unit to succinic anhydride is 0.8), and the elemental analysis degree of substitution is 0.77mol / unit. The activated partial thromboplastin time and thrombin time reached 0.5 times and 0.9 times of the corresponding chitosan sulfate respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com