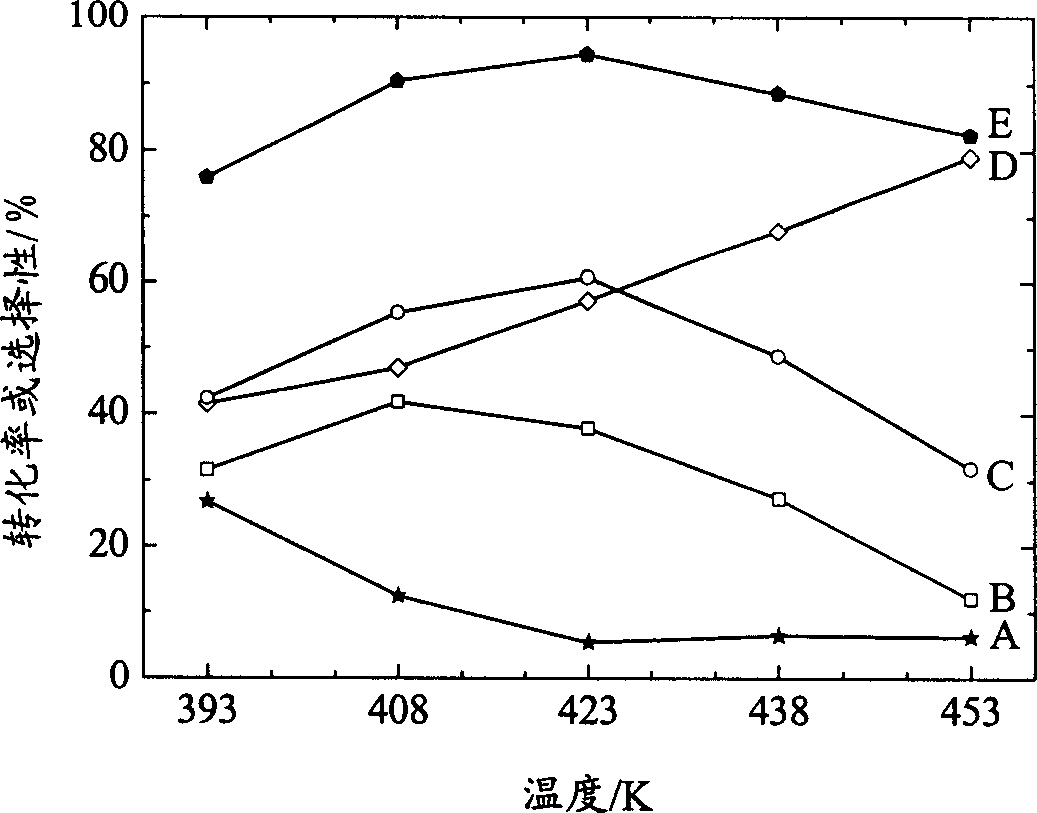
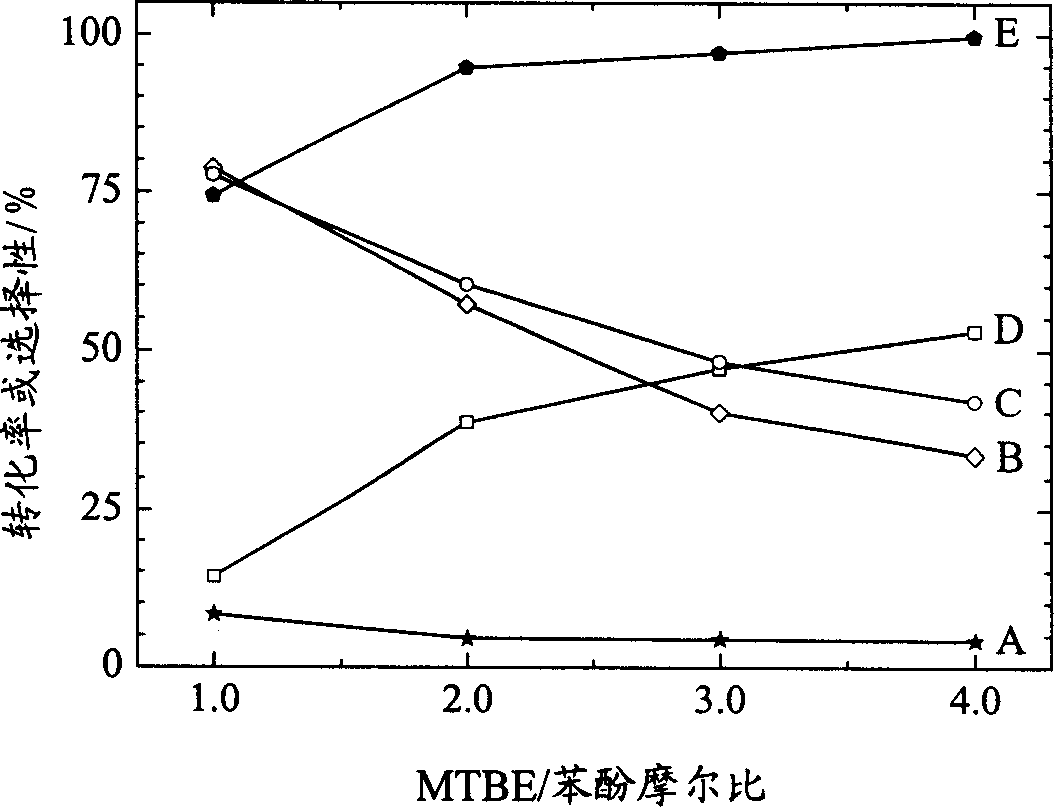
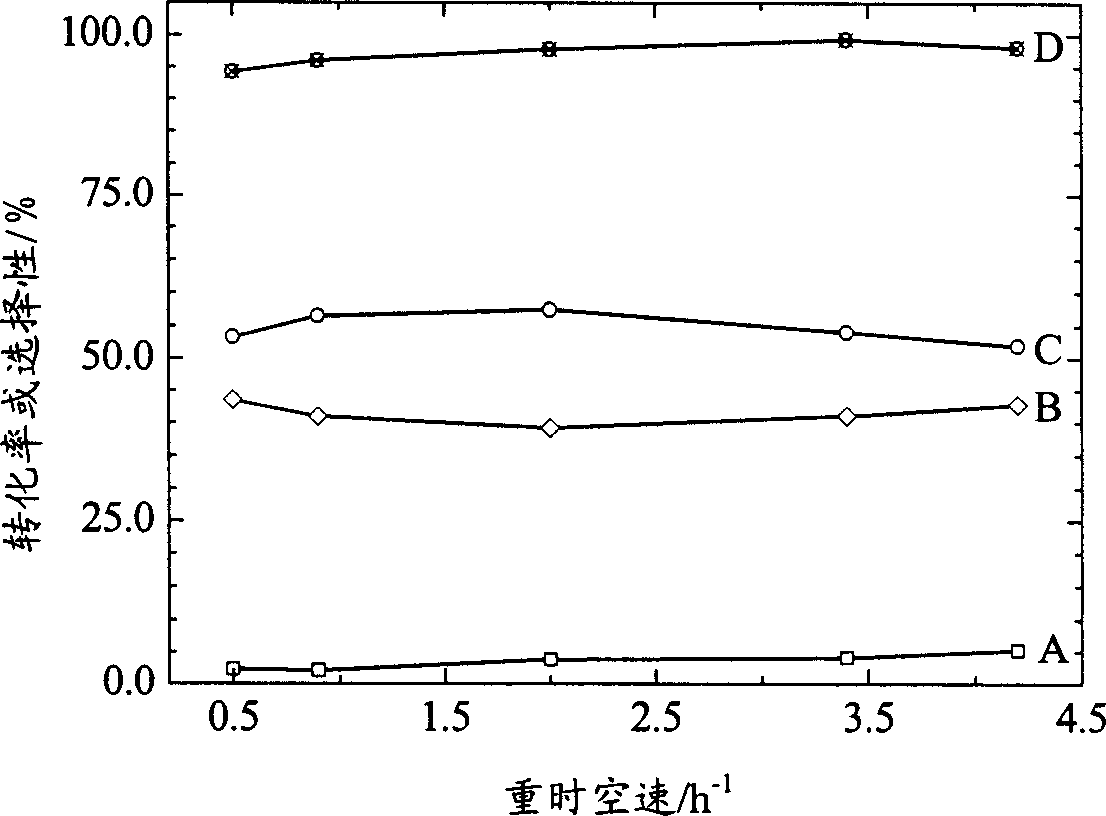
Novel catalyst for synthesizing tertiary-butyl substituted benzene phenols and its preparation process
A technology of tert-butyl group and catalyst is applied in the field of a new method for synthesizing tert-butyl substituted phenol and related solid acid catalysts, which can solve problems such as no industrial application results, and achieves convenient product separation and purification, simple process and convenient preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Get 0.1kg sodium type MFI microporous structure crystalline aluminosilicate (SiO 2 / Al2 o 3 =55) Cooled after 3 hours of roasting at 1123K, and 4.0×10 -4 m 3 The concentration is 1.0×10 2 mol / m 3 Mix the hydrochloric acid solution, stir at 368K for 1h, filter after cooling, wash the solid with distilled water until no chloride ions are detected in the filtrate, and mix the same hydrochloric acid solution with the solid, repeat the above operation for a total of 4 times, and the obtained solid is dried at 823K Calcined for 4 hours, the catalyst carrier HMFI with 0.2% sodium residue was obtained.
[0024] Take the above catalyst carrier HMFI 5g, 3.54×10 -4 kg Al(NO 3 ) 3 9H 2 O soluble in 4.0×10 -6 m 3 impregnated in distilled water at room temperature, dried at 393K for 2 hours, and roasted at 823K for 3 hours to obtain Al 2 o 3 Catalyst Al-MFI (1.0) at a content of 1.0%.
Embodiment 2
[0026] Take the catalyst Al-MFI (1.0) prepared according to the method of Example 1, press it into tablets, crush it into 20-30 meshes, take 1 g and put it into a fixed-bed continuous flow reaction device. The molar ratio of phenol and isobutylene is 1:1.2, and the weight hourly space velocity is 2.5h -1 Import the front-end device to preheat at 393K, then flow through the catalyst Al-MFI (1.0) with a bed temperature of 393K, and analyze the product with an on-line reaction time of 3 hours. The results are as follows: phenol conversion rate 37.2%; phenol alkylation The o-TBP selectivity in the product was 19.2%, the p-TBP selectivity was 57.8%, and the 2,4-DTBP selectivity was 23.0%.
Embodiment 3
[0028] Get 0.2kg sodium type BEA microporous structure crystalline aluminosilicate (SiO 2 / Al 2 o 3 =24) Cooled after 3 hours of roasting at 1023K, with 1.0×10 -3 m 3 The concentration is 4.0×10 2 mol / m 3 Mix the hydrochloric acid solution, stir at 363K for 1h, filter, wash the solid with distilled water until no chloride ions are detected in the filtrate, and mix the same hydrochloric acid solution with the solid, repeat the above operation for a total of 5 times, and the obtained solid is dried and roasted at 823K for 3 hours, the catalyst carrier HBEA-1 with a residual sodium content of 0.05% was obtained.
[0029] Take the above catalyst carrier HBEA-110g, 3.54×10 -4 kg Al(NO 3 ) 3 9H 2 O soluble in 7.5×10 -6 m 3 impregnated in distilled water at room temperature, dried at 393K for 3 hours, and roasted at 823K for 3 hours to obtain Al 2 o 3 Catalyst Al-BEA-1 (0.5) at 0.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com