Poly-aryl ether ketone performed polymers terminated with phenyl-ethynyl, preparation method therefor and crosslinked materials using the same
A technology of polyaryletherketone and phenylacetylene, which is applied in the field of polyaryletherketone prepolymers to prepare high-performance cross-linked materials, can solve the problems of high processing temperature, difficulty, and low use temperature, and achieve excellent processing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
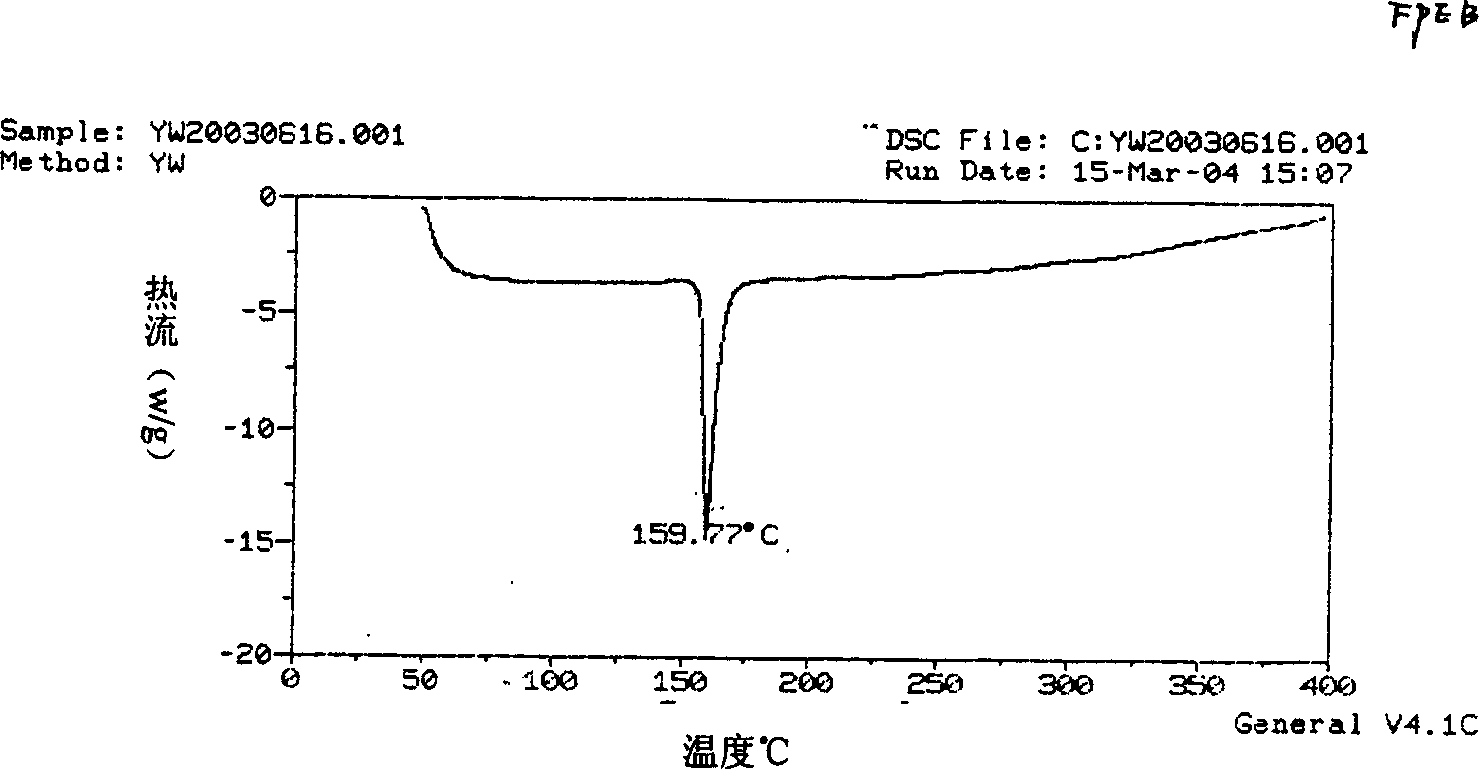
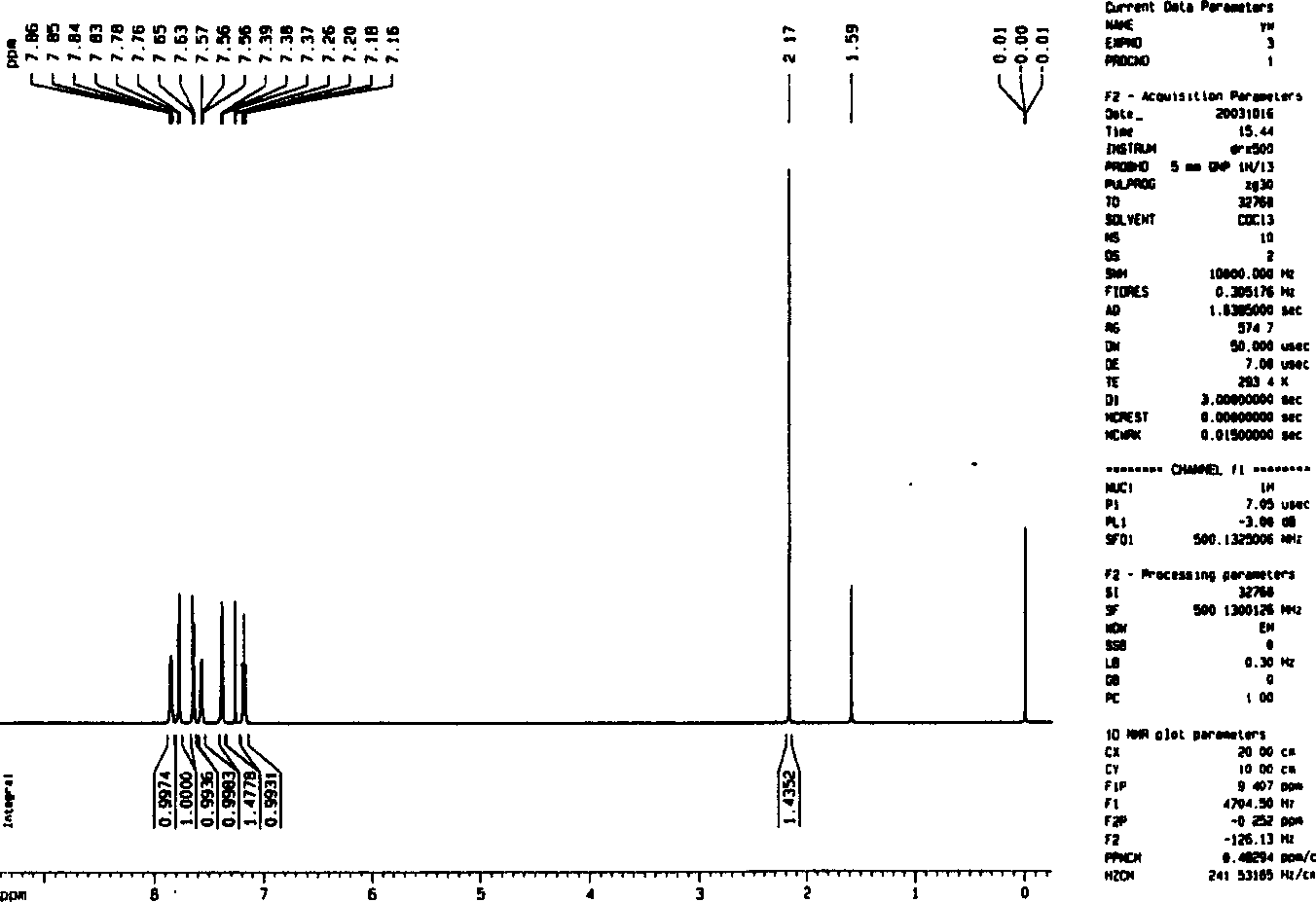
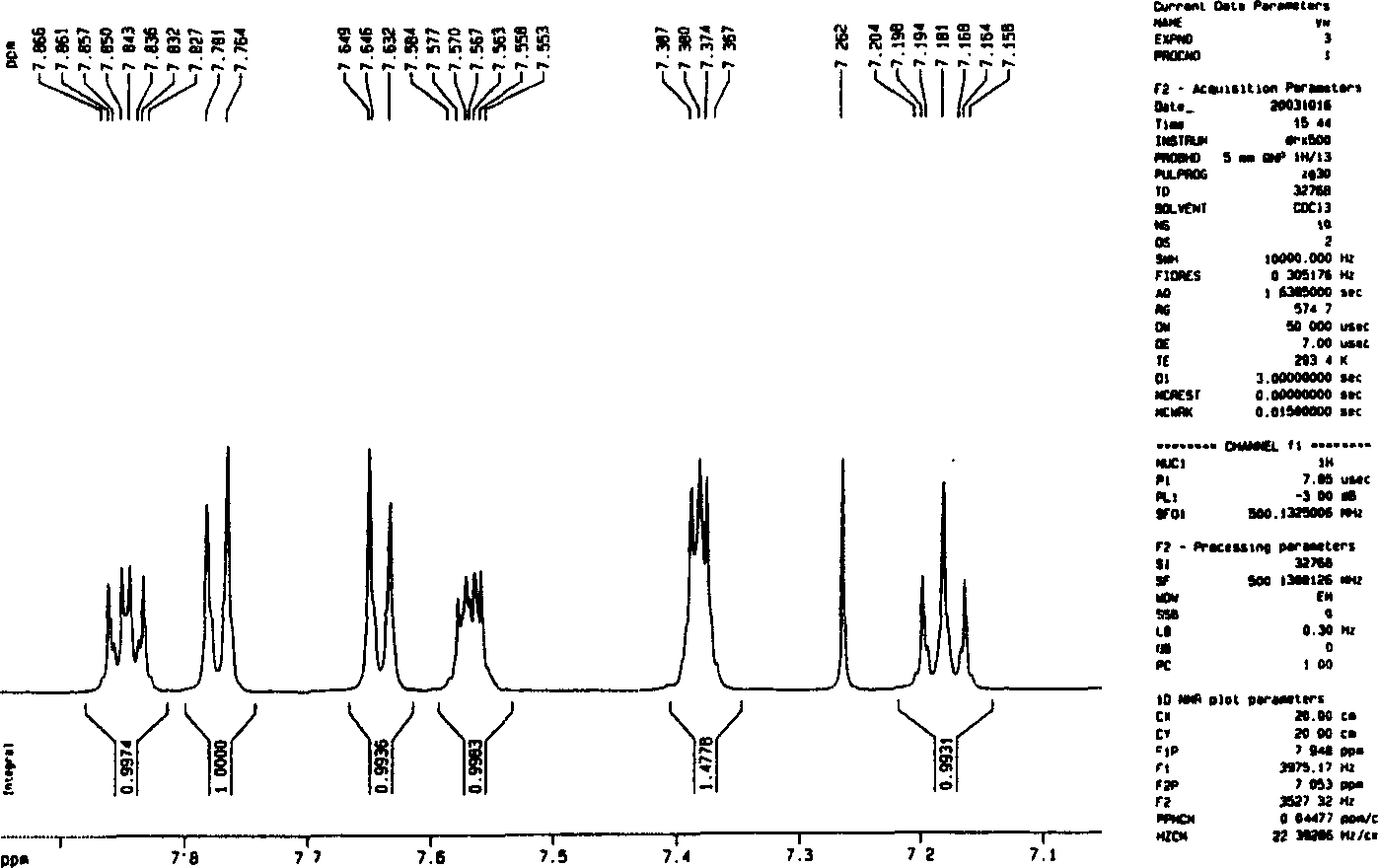
Image
Examples
Embodiment 1
[0053] Add 39.8g (0.18mol) of p-bromobenzoyl chloride and 135ml of fluorobenzene in an 8-fold excess to a three-necked flask equipped with mechanical stirring, reflux with water device, hydrogen chloride gas absorption device and nitrogen inlet and outlet. After cooling in an ice bath to 0°C, add 33.8g (0.25mol) of catalyst anhydrous aluminum trichloride, rise to room temperature and stir for about 15 minutes, then heat to reflux temperature and stir for 3 to 6 hours. After cooling to room temperature, the mixture was stirred for 6-16 hours and the reaction was completed. After the reaction is over, add 20-50ml of distilled water dropwise at a rate of 1 drop per second to remove excess aluminum trichloride, remove excess fluorobenzene with a fluorobenzene / water azeotropic system, and then use 10% by weight NaOH base The solution was boiled and washed twice, and further boiled and washed with distilled water three times until the filtrate was neutral, dried, and recrystallized ...
Embodiment 2
[0056] 1.008 g of triphenol monomer (0.008 mol) was dissolved in 30 ml of DMAC. In the 250ml three-neck flask equipped with mechanical stirring, reflux with water device and nitrogen inlet and outlet, add 7.2g of fluorine-containing alkyne monomer (0.024mol), 2.5g of catalyst anhydrous potassium carbonate, 150ml of DMAC and 40ml of toluene, After the system was heated to reflux with water for 2 hours, the pre-prepared DMAC solution of triphenol monomer was dropped into the reaction system at a rate of 4 seconds / drop, and the reflux was kept with water, and the reaction was continued for 24 hours after the dropwise addition. The reaction solution was discharged into 2000ml of HCl aqueous solution, and after standing still, the dark yellow precipitate was filtered, dried, washed twice with ethanol, dried in an oven, and the obtained crude product was recrystallized by DMAC to obtain phenylacetylene-terminated branch pre The milky white product of the aggregate was 6.80 g (yield:...
Embodiment 3
[0058] Contrary to the order of addition of raw materials in Example 2, other embodiments are the same, thus causing a very high concentration of triphenols at the moment when the alkyne monomer is dripped in: 7.2g of fluorine-containing alkyne monomer (0.024mol) is dissolved in 80ml of In DMAC, pre-add 1.008g triphenol monomer (0.008mol), 2.5g of catalyst anhydrous potassium carbonate, 150ml of DMAC and 40ml of toluene in a 250ml three-neck flask equipped with mechanical stirring, reflux with water device and nitrogen inlet and outlet After the system was heated to reflux with water for 2 hours, the DMAC solution of the pre-configured triphenol monomer was dropped into the reaction system at a speed of 3 seconds / drop, and the reflux was kept with water, and the reaction was continued for 12 hours after the dropwise addition. The reaction solution was discharged into 2000ml of HCl aqueous solution, and after standing still, the dark yellow precipitate was filtered, dried, washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com