Metal micro needles array chip and preparation method, and usage
A technology of microneedle array and metal substrate, which is applied in other medical devices, drug devices, drug devices, etc., can solve the problems of restricting metal microneedles, and achieve the effect of simple process and good durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
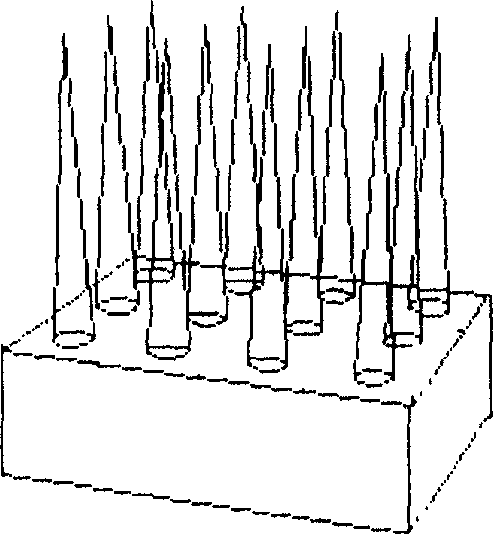
[0042] Embodiment 1, multi-row metal microneedle array (out-of-plane)
[0043] See Figure 3.
[0044] a) A stainless steel sheet 1 with a thickness of 500 microns is washed with deionized water and dried after being cleaned with lotion. Then use chemical vapor deposition to deposit a layer of silicon dioxide 2 with a thickness of 1 micron on both surfaces of the metal as a protective layer for the metal, and spin-coat Shipley 1818 photoresist 3 with a thickness of 2 microns on the silicon dioxide on one side. , (shown in 3a);
[0045] b) Pre-bake (soft bake) at 90°C for 5 minutes, and form a 20×20 array of solid circles with a diameter of 80 microns and a pitch of 200 microns in a unit of 4 square millimeters. The substrate with photoresist is exposed for 4 seconds, then developed and dried at 120°C for about 30 minutes (see 3b); see the top view for the effect prepared by the steps;
[0046] c) Spin-coat Shipley 1818 photoresist 3 with a thickness of 2 microns on the silic...
Embodiment 2
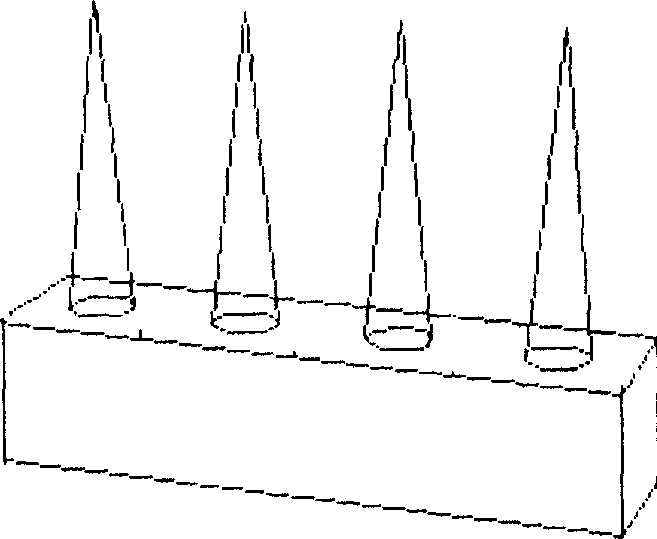
[0052] Embodiment 2, single-row metal microneedle array (in-plane) manufacturing process
[0053] See Figure 4 .
[0054] a) After the 100 micron thick titanium sheet 1 is cleaned with lotion, it is rinsed with deionized water and dried; Silicon 2 is used as a protective layer of metal, and a 2-micron thick Shipley 1818 photoresist 3 is spin-coated on the silicon dioxide surface on one side of the substrate, (see 4a);
[0055] b) Pre-bake (soft bake) at 90°C for 5 minutes, cover the substrate with photoresist with a quartz glass mask with a microneedle pattern and expose it for 4 seconds, then develop and dry at 120°C for about 10 minutes; Spin-coat Shipley 1818 photoresist 3 with a thickness of 2 microns on the silicon dioxide surface on the other side of the substrate, pre-bake (soft bake) at 90°C for 5 minutes, and cover the photoresist with the quartz glass mask used in the previous step. Align and expose both sides of the glue for 4 seconds, then develop and dry at 12...
Embodiment 3
[0059] Embodiment 3, taking bovine serum albumin as the rat in vitro transdermal drug release of model drug
[0060] Dissolve bovine serum albumin in 0.25% carbomer (pH 6.5) solution to prepare a mixed solution containing 25mg / ml bovine serum albumin, get 40 microliters and add it to the surface of the microneedle prepared in Example 1 and dry it naturally at room temperature to form a mixture containing Polymer film microneedle preparation of bovine serum albumin. The microneedle has 400 needles in an area of 4 square millimeters, with a pitch of 200 micrometers between the needles.
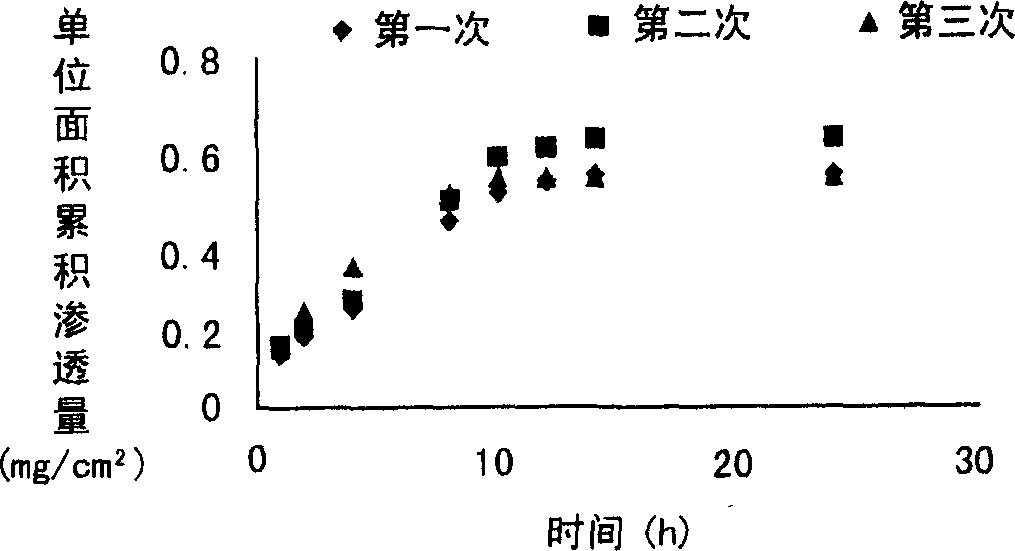
[0061] Take a piece of hair-free rat skin, put the cuticle up at the mouth of the diffusion pool, place the solid microneedle prepared above on it, press the microneedle lightly with your hand to make micropores on the skin, but do not penetrate the skin, The stratum corneum of the skin faces the drug supply chamber, and the dermis faces the drug receiving chamber. Fill the receiving chamber ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com