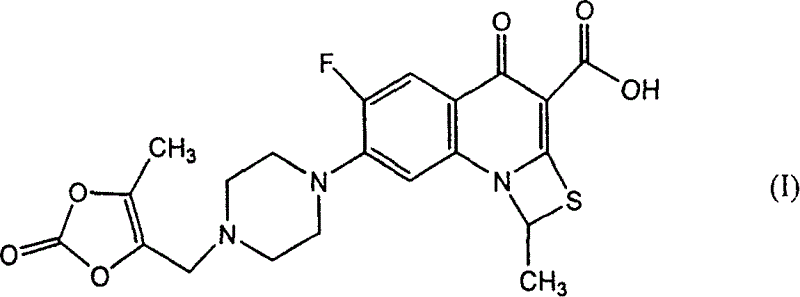
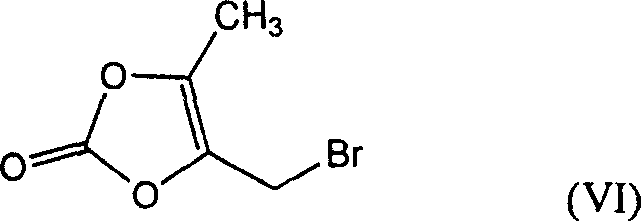
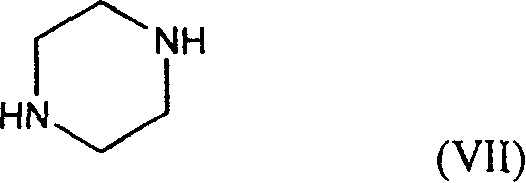Production of plurizacin
A compound, methyl technology, applied in the production field of prulifloxacin, can solve the problems of complex processing, low purity, and difficulty in obtaining, etc., and achieve the effect of simple process, high yield, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, preparation of 6,7-difluoro-1-methyl-4-oxidation-4H-[1,3]thiazetidin[3,2-a]silaline-3-carboxylic acid
[0034] Add 36g of 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazetidin[3,2-a]silaline-3-carboxylate successively to a 500ml three-necked flask Ethyl ester, 200ml glacial acetic acid, 30ml concentrated hydrochloric acid, 20ml water, heat and reflux for 12 hours, after the reaction is complete, concentrate under reduced pressure to dryness, add 500ml ice water, a white solid precipitates, filter with suction, wash with 50ml X2 ice water, vacuum After drying at 60°C, 26.5 g of white solid powder was obtained as Intermediate III, with a yield of 94%.
Embodiment 2
[0035] Embodiment 2, preparation 5-methyl-4-piperazine methyl-1,3-dioxoledione
[0036] In the 500ml there-necked flask, add 34.4g anhydrous piperazine successively, 100mlDMF, stir at room temperature until dissolving, add dropwise the DMF solution of 4-bromomethyl-5-methyl-1,3-dioxolenedione ( 19.3g / 50ml), continue to react at room temperature for 2 hours after dropping, filter after the reaction, add 1000ml ice water to the filtrate, extract with 250ml×2 chloroform, wash the combined organic layer with 500ml×3 ice water, dry everything, concentrate under reduced pressure to nearly dry 18 g of red solid was obtained as the crude compound of formula (II). Refining: Recrystallize with ethyl acetate-petroleum ether to obtain a light red solid, mp=87.5-90°C; quality control standard: TLC developer: methanol: chloroform: triethylamine = 1:3:0.1, after thin chromatography , dried at 60°C for 1 hour, developed with iodine-potassium iodide-bismuth potassium nitrate, Rf=0.3.
[0037...
Embodiment 3
[0043] Embodiment 3, preparation prulifloxacin
[0044] In the 500ml there-necked flask, add 30g potassium bicarbonate, 200mlDMF successively, stir for 10 minutes, add 14.1g formula (III) compound, stir at room temperature for 1 hour, then add 18 formula (II) compound, react for 3 hours at 50 DEG C, TLC monitoring ( Developing solvent: ammonia form: methanol = 92.8), the compound of formula (III) basically disappears as the reaction end point. After the reaction was complete, 1000ml of ice water was added, and a white solid precipitated out. It was suction filtered, washed with a large amount of ice water, and dried in vacuum at 25°C to obtain 21.2 g of off-white solid as crude prulifloxacin.
[0045] Refining: 21.2 prulifloxacin crude product, dissolved in 400ml acetonitrile at 50°C, cooled to room temperature, left standing overnight, a large amount of solids precipitated, filtered with suction, washed with 30ml×2 cold acetonitrile, dried in vacuum at 20°C 17.8 fine pruliflo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com