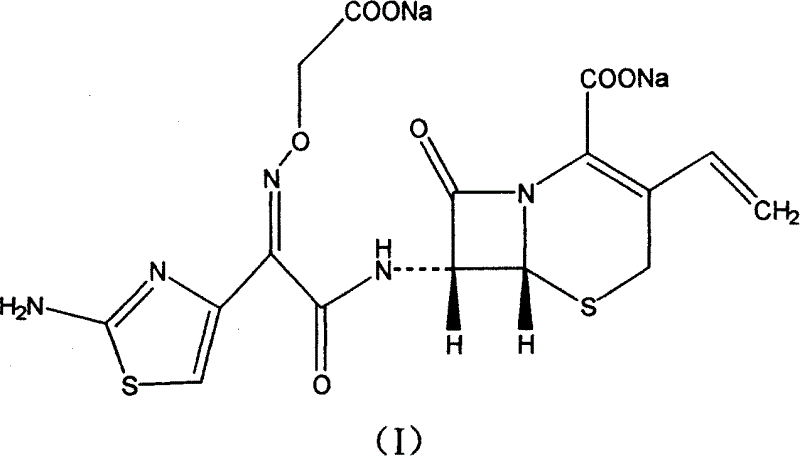
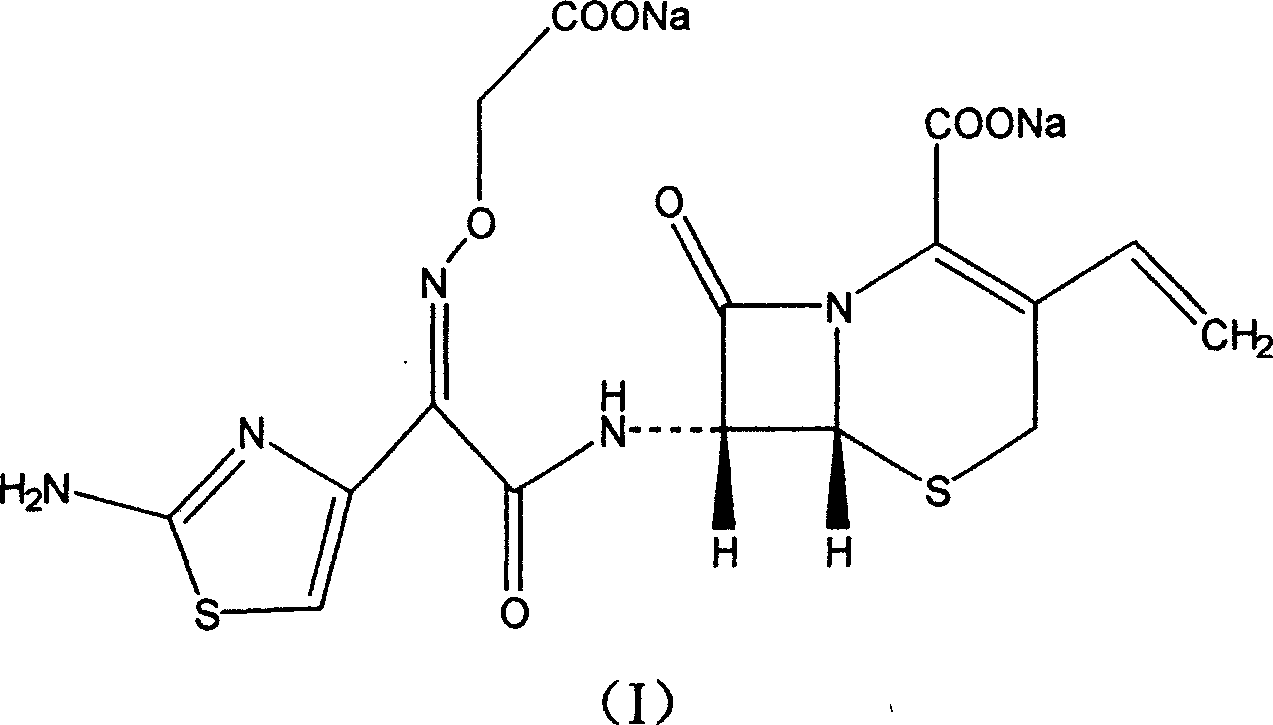
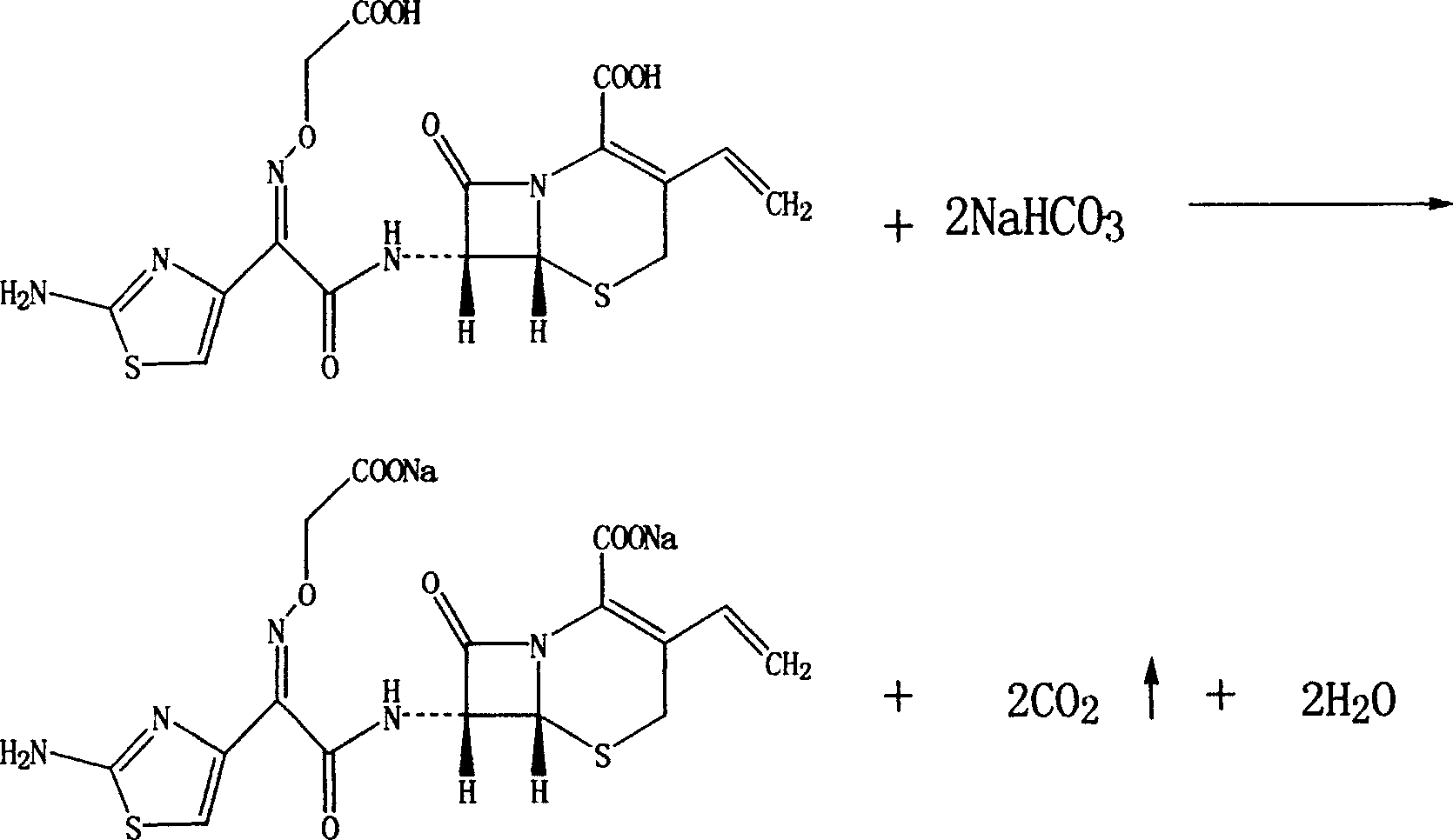
Cefixime sodium pharmaceutical composition and its preparation and application
A pharmaceutical compound, cefixime technology, applied in the field of cefixime sodium pharmaceutical compounds, can solve the problems of poor water solubility of cefixime and the inability to prepare injections, etc., and achieve good water solubility, low cost and high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the preparation of cefixime sodium
[0016] Under normal temperature and pressure, 507.5 grams of cefixime and 1680 grams of 10% aqueous sodium bicarbonate were added to the reaction flask, stirred and reacted for 2 hours, 10 grams of active carbon for needles was added, stirred for 20 minutes, filtered, and the filtrate was crystallized with ethanol. Suction filtration, vacuum drying at 50°C to obtain the primary product of cefixime sodium. Under aseptic conditions, recrystallize with ethanol aqueous solution, and vacuum-dry at 50°C to obtain 427 grams of cefixime sodium finished product.
[0017] Yield: 85.9%
[0018] Appearance: off-white crystalline powder
[0019] Content: 99.1%
[0020] PH: 7.2 (10% aqueous solution)
Embodiment 2
[0021] Embodiment 2: Preparation of Cefixime Sodium Powder for Injection
[0022] The cefixime sodium raw material is prepared and subpackaged according to the sterile powder injection process to obtain the cefixime sodium powder injection for injection.
Embodiment 3
[0023] Embodiment 3: Preparation of cefixime sodium freeze-dried powder for injection
[0024] Prescription Cefixime Sodium 500g
[0025] 5% sodium bicarbonate solution appropriate amount
[0026] Add water for injection to 1000ml
[0027] Preparation method Weigh 500 grams of cefixime sodium in a sterile operating room, put it in a sterile container, add sterile water for injection to about 500 ml, stir to dissolve, add 5% sodium bicarbonate solution to adjust the pH to 6.8-7.2 Within the range, add water for injection to a sufficient amount, then add 2% of the prepared activated carbon for needles, stir for 5-10 minutes, pre-filter, fine filter, sub-package, pre-freeze for about 2 hours, freeze-dry at low temperature for 24 hours, Add stopper and roll the cap to get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com