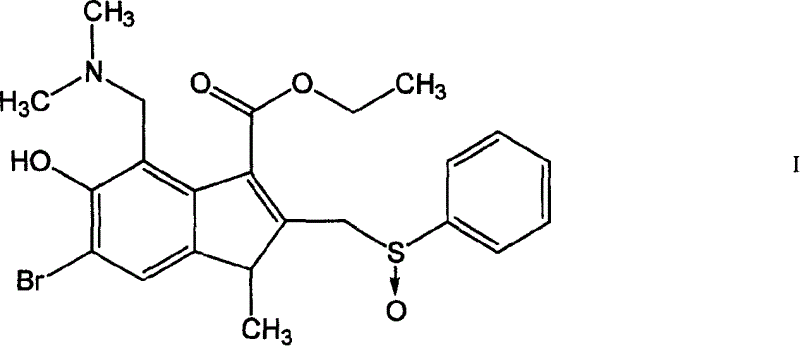
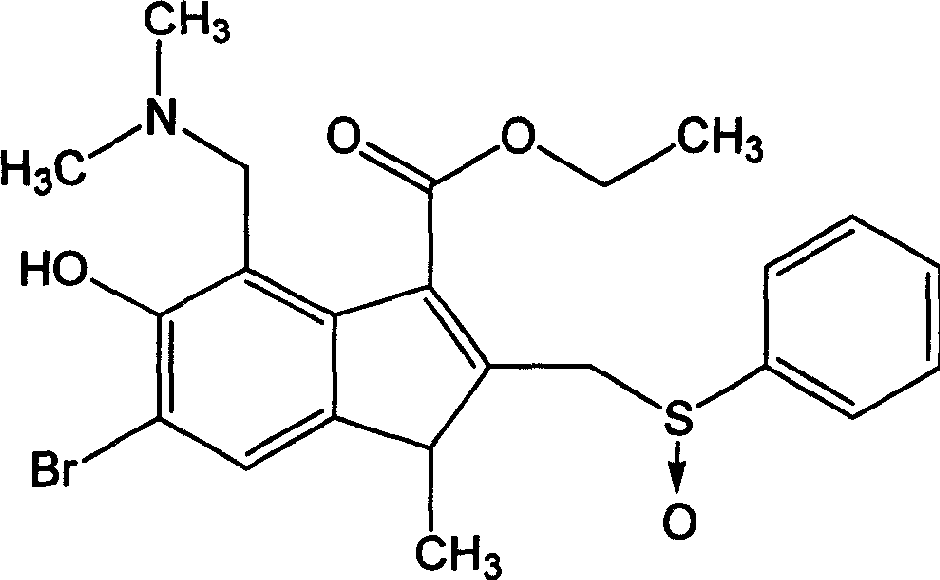
Application of albi multisulfoxide and its salt in preparing medicine for preventing and treating respiratory tract virus infestation
A technology for virus infection and drug treatment, applied in the field of medicine, can solve problems that have not yet been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: In vitro anti-influenza virus experimental research of Albidol hydrochloride
[0020] 1. Materials and methods
[0021] 1. Virus: Influenza virus type A (H 1 N 1 and H 2 N 2 ) and type B were all quoted from the Institute of Virology, Chinese Academy of Preventive Medicine; influenza virus clinically isolated type A virus strains were provided by the Hebei Provincial Health and Epidemic Prevention Station; after the virus was inoculated with chicken embryos to increase the virus, the allantoic fluid was collected and stored at -70°C for later use.
[0022] 2. MDCK cell line: quoted from China Institute for the Control of Pharmaceutical and Biological Products.
[0023] 3. Drugs:
[0024] (1) Positive control drug: ribavirin (RBV), product of Hubei Keyi Pharmaceutical Factory, batch number (010913).
[0025] (2) Experimental drug: Albidol sulfoxide hydrochloride, provided by the Institute, batch number (020101).
[0026] 4. Drug preparation: dissolve in...
Embodiment 2
[0173] Embodiment 2: Preparation of Albidosulfoxide Hydrochloride Sodium Chloride Injection
[0174] (1) In the aseptic operation room, weigh 49.18g (0.036mol) of hydroxypropyl-β-cyclodextrin, then add 525mg of tartaric acid, add water to dissolve to 900ml, add 1g of activated carbon for infusion, stir and heat to 80°C, keep warm After 15 minutes, decarbonize by filtration.
[0175] (2) Weigh 10 g (0.018 mol) of albidol sulfoxide hydrochloride and add it into the hydroxypropyl-β-cyclodextrin solution.
[0176] (3) The liquid was mixed by magnetic stirring for about 20 minutes, and the solution gradually became clear, and the Albido sulfoxide hydrochloride-hydroxypropyl-β-cyclodextrin inclusion compound solution was obtained.
[0177] (4) Weigh 75g of sodium chloride for injection, add water to 1L, stir to dissolve, add 1g of activated carbon for infusion, heat to a slight boil for 15 minutes, and decarbonize.
[0178] (5) Pour the sodium chloride solution into the clathrate ...
Embodiment 3
[0180] Embodiment three: the preparation of albidol sulfoxide hydrochloride tablet
[0181] 1 recipe:
[0182] Albidosulfoxide Hydrochloride 100mg
[0183] Starch 83mg
[0184] Microcrystalline Cellulose 167mg
[0185] Cross-linked polyvinylpyrrolidone 15mg
[0186] 10% polyvinylpyrrolidone 80% ethanol appropriate amount
[0187] the solution
[0188] Sodium Lauryl Sulfate 2.5mg
[0189] Magnesium Stearate 2.5mg
[0190] Tablet weight about 400mg
[0191] 2Operation steps:
[0192] (1) Pass albidol sulfoxide hydrochloride through an 80-mesh sieve, weigh the prescription amount and mix it evenly with starch, microcrystalline cellulose and cross-linked polyvinylpyrrolidone.
[0193] (2) Use 15% polyvinylpyrrolidone and 85% ethanol solution to make soft materials, granulate with a 20-mesh sieve, dry at 50-55°C, and granulate with a 18-mesh sieve.
[0194] (3) Add sodium lauryl sulfate and magnesium stearate according to the weight of dry granules, and then compress into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum non-toxic concentration | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com