Eugenol ibuprofen ester medical compound and its preparation and preparation method
A technology of ibuprofen axetil and eugenol ester, applied in the field of medicine, can solve problems such as unstable placement at room temperature, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
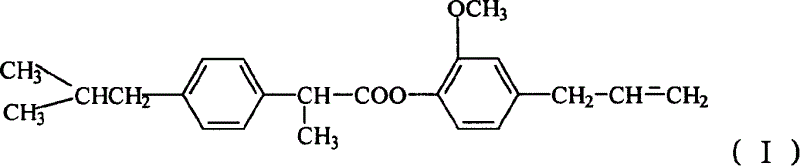
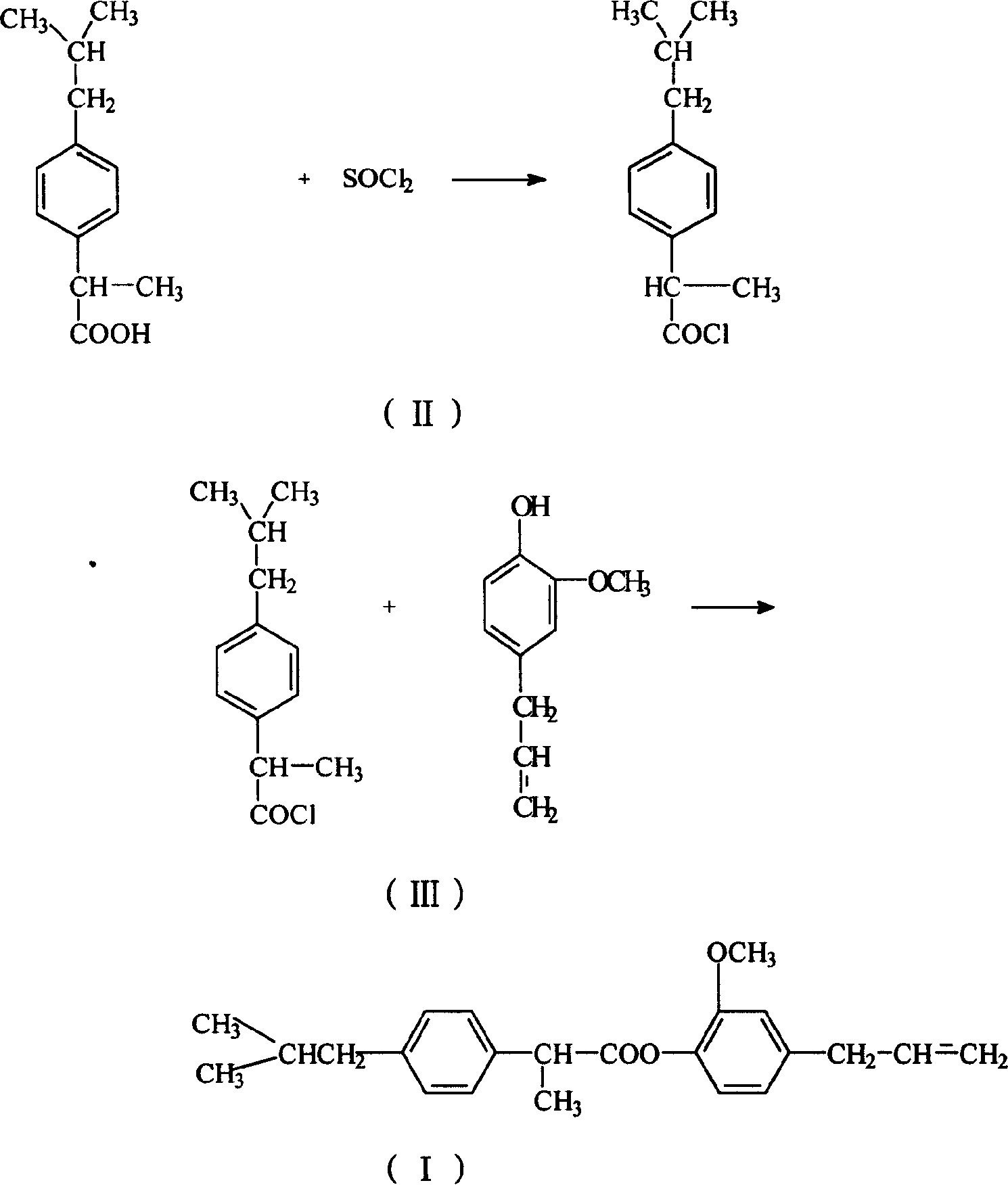
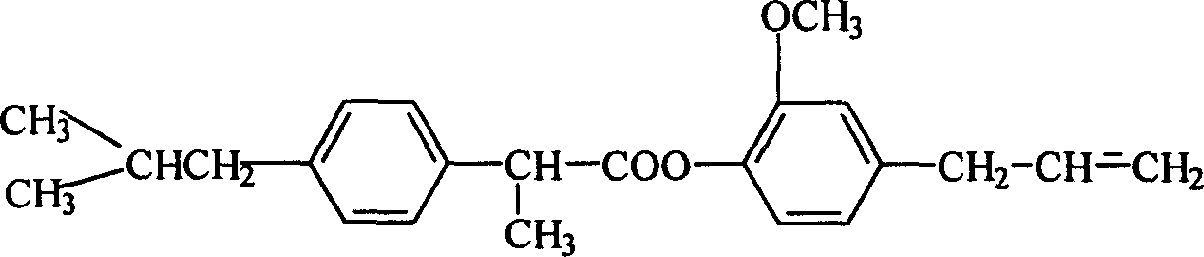
[0009] The preparation route of eugenol ibuprofen ester is as follows:
[0010]
[0011] Add ibuprofen and thionyl chloride to a 50 ml three-neck flask equipped with a stirrer and a thermometer, stir and react at 50-80°C for 1-2 hours, evaporate excess thionyl chloride under reduced pressure, and distill the acid chloride Dissolve in acetone and / or ethyl acetate solution containing anhydrous potassium carbonate or anhydrous magnesium sulfate, add eugenol with an addition funnel (make the molar ratio of eugenol and thionyl chloride 1:1-1.6), at room temperature React for 3-8 hours, filter, recover acetone or ethyl acetate, dissolve the concentrated solution in ethyl acetate, wash 1-3 times with 5%-10% sodium hydroxide solution, wash 1-3 times with water, and use the extract Anhydrous magnesium sulfate was dried overnight, and after recovering the solvent, it was recrystallized with petroleum ether to obtain light yellow solid crystals with a yield of 60%. The melting point ...
Embodiment 1
[0028] Prescription: Eugenol 25mg
[0029] Migylol 812 40-55mg
[0030] Phospholipids 20-30mg
[0031] HS-15 20-30mg
[0032] Absolute ethanol 10-30mg
[0033] 1,2-propanediol 10-30mg
[0034] water 2-3ml
[0035] Preparation method: ①Dissolve eugenol ester in Migylol812 containing absolute ethanol, 1,2-propanediol, phospholipid, and HS-15, and stir overnight at 37°C until equilibrated.
[0036] ②Titrate the oil phase with water to form a uniform and stable eugenol ester microemulsion.
Embodiment 2
[0038] Prescription: Eugenol 25mg
[0039] Phospholipids 40-60mg
[0040] Cholesterol 10-40mg
[0041] Chloroform
[0042] water 2-3ml
[0043] Preparation method: ① Dissolve eugenol ester in chloroform solution containing phospholipids and cholesterol, and remove chloroform by rotary evaporation to form a phospholipid film.
[0044] ② Dissolve the phospholipid membrane with water, and form uniform liposomes by crushing cells and ultrasonically.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com