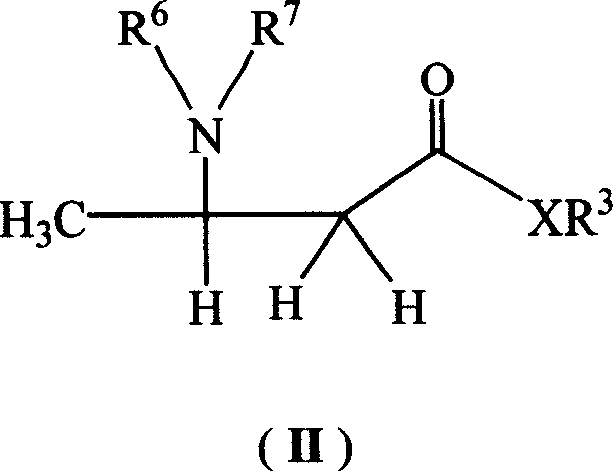
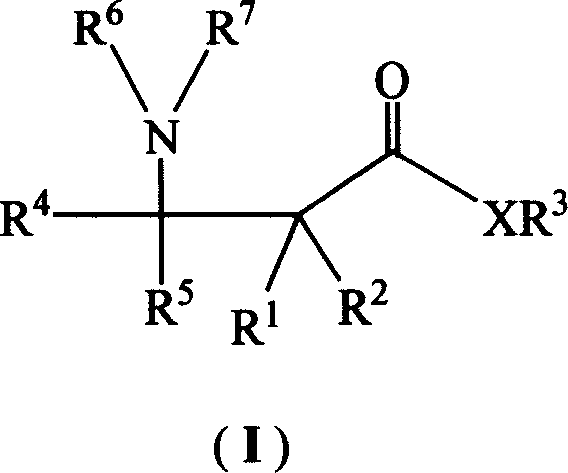
DL-beta-amino butyric acid derivative and its preparing method and use
A technology of aminobutyric acid and derivatives, which is applied in the field of aminobutyric acid derivatives, and can solve the problems of low induction activity and large dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
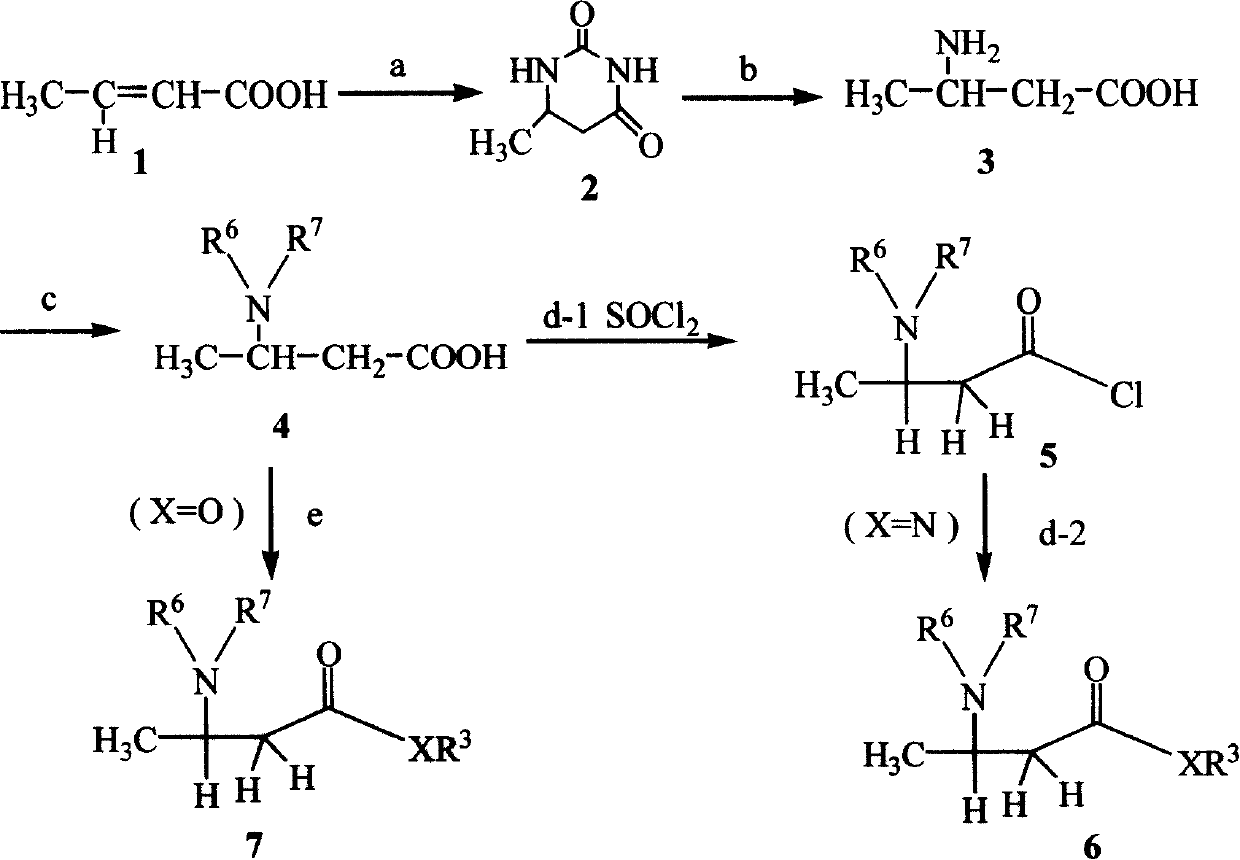
[0047] Step 1, the synthesis of 2-methyl-6-hydrouracil:
[0048] Take 43g of crotonic acid (1), 75g of urea, and 150mL of ethylene glycol, heat to 190°C under stirring, keep the temperature at 185°C to 195°C, and reflux for 1 hour. After the reaction is complete, cool the solution and put it in the refrigerator Freeze overnight until a large amount of white solids appear, filter, wash with cold ethanol and a small amount of ice water, dry the white solids obtained, and then recrystallize in ethanol to obtain the product 2-methyl-6-hydrouracil ( 2);
[0049] Step 2, synthesis of DL-β-aminobutyric acid:
[0050] Add 2.56g of 2-methyl-6-hydrouracil (2) into the reaction flask, add 8g of sodium hydroxide and 200mL of water, and heat to reflux for 8 hours under mechanical stirring. After the reaction is completed, the solution is cooled and passed through 50-100 mesh cation exchange resin column, carbon dioxide gas is released at the beginning, rinse with about 800mL of distilled...
Embodiment 2~17
[0058] The 1st~4 steps, with embodiment 1;
[0059] Step 5, except that the aniline in Example 1 is replaced by phenethylamine, o-chloroaniline, m-chloroaniline, 2,4,5-trichloroaniline, m-bromoaniline, p-bromoaniline, o-methylaniline, m-methylaniline, o-methoxyaniline, p-fluoroaniline, 2,3,4-trifluoroaniline, 2-aminothiazole, 2-amino-1,3,5-thiadiazole, 3-amino-5- Except methylpyrazole, 3-amino-5-phenyl-1,2,4-triazole or 3-methylthio-4-cyano-5-aminopyrazole, other are the same as embodiment 1, respectively get Products and their yields are shown in II-1b, II-1c, II-1d, II-1e, II-1f, II-1g, II-1h, II-1i, II-1j, II-1k, II-1l, II-1m, II-1n, II-1o, II-1p, II-1q.
Embodiment 18
[0061] The 1st~4 steps, with embodiment 1;
[0062] The 5th step, the synthesis of 2-N-benzothiazole-3-N-benzyloxycarbonyl-aminobutanamide (II-1r):
[0063]Dissolve 1.5 mmol of 2-aminobenzothiazole in 8 mL of tetrahydrofuran, add 0.23 mL of triethylamine, cool in an ice-water bath, and add 1 mmol of 3-benzyloxycarbonyl-β-aminobutyryl chloride (5) dropwise at 0°C 6mL tetrahydrofuran solution, white insoluble matter was formed, which was triethylamine hydrochloride, monitored by TLC, reacted for 0.5 to 1 hour, filtered, evaporated the solvent under reduced pressure, added 20mL chloroform to dissolve the residue, washed with 1mol / L hydrochloric acid , washed with water, dried with anhydrous sodium sulfate, removed the solvent, separated by vacuum column chromatography to obtain the product or recrystallized with toluene to obtain the product 2-N-benzothiazole-3-N-benzyloxycarbonyl-aminobutyramide (II -1r) 0.23 g, yield 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com