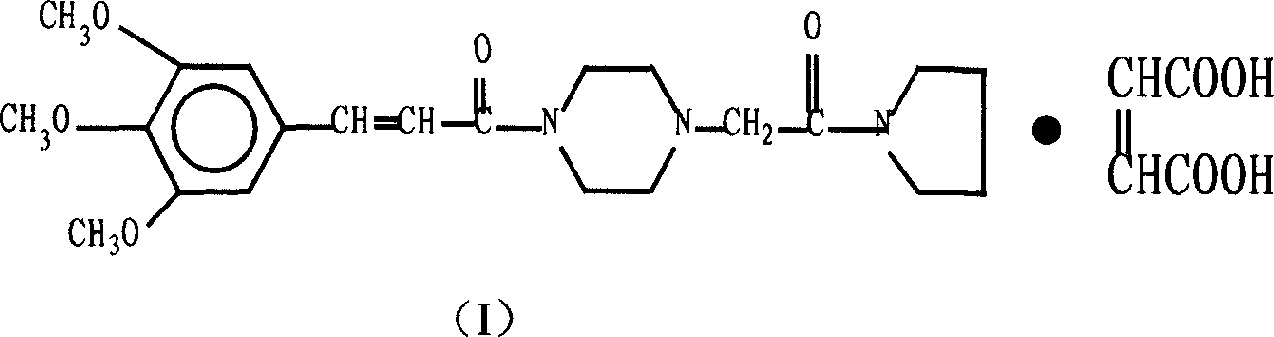
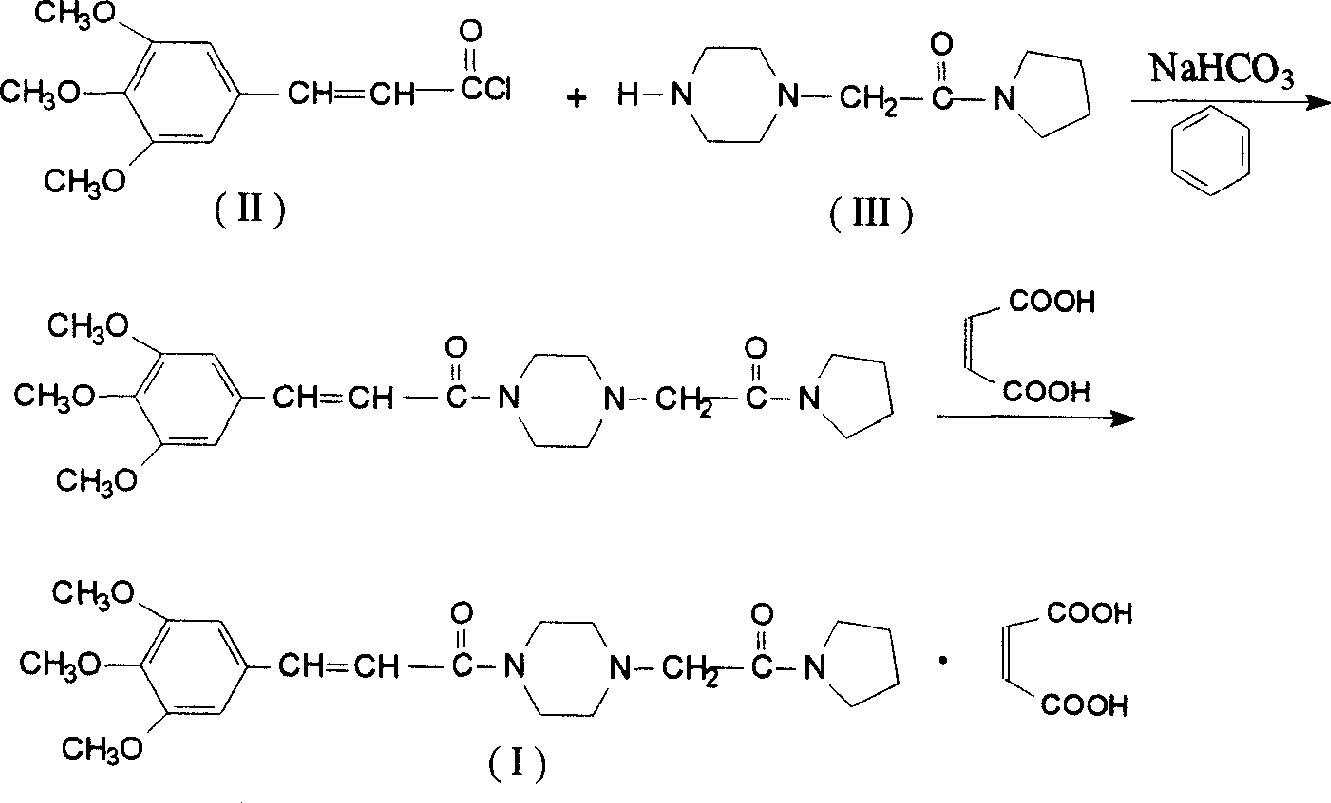
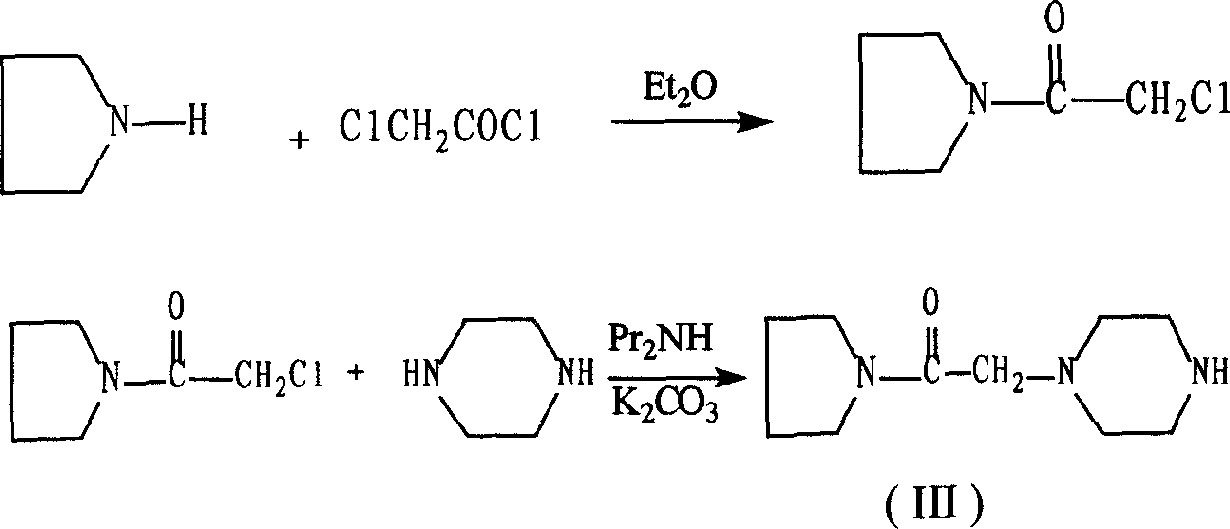
Synthesis method of cinepazide maleate
A technology for the synthesis of cinepazide maleate and a synthesis method, which is applied in the field of synthesis of cinepazide maleate, can solve the problems of high production cost, low boiling point of ether, high requirements for production conditions and equipment, and achieve Simplify production conditions and process, high product yield, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment 1 cinepazide maleate:
[0049] 1) synthesis of chloroacetylpyrrolidine
[0050] Reaction formula:
[0051]
[0052] operate:
[0053] Slowly add 300 g of tetrahydropyrrole into a solution of 230 g of chloroacetyl chloride in 1,400 g of dichloromethane under stirring at 10°C. After the addition, continue to stir at room temperature for 2 hours, heat and recover the dichloromethane, and wash the residue with 300 g of ethyl acetate. Dissolve the ester, add 1400g of petroleum ether at 60-80°C, and cool. Tetrahydropyrrole hydrochloride crystallized out, recovered by filtration, and reused after alkalization. The filtrate was washed with 400ml of saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a colorless liquid, which solidified at room temperature to obtain 492g of chloroacetylpyrrolidine, mp. 40-46°C, yield 80%.
[0054] 2) 1-piperazine acetylpyrrolidine:
[0055] Reaction formula:
[0056] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com