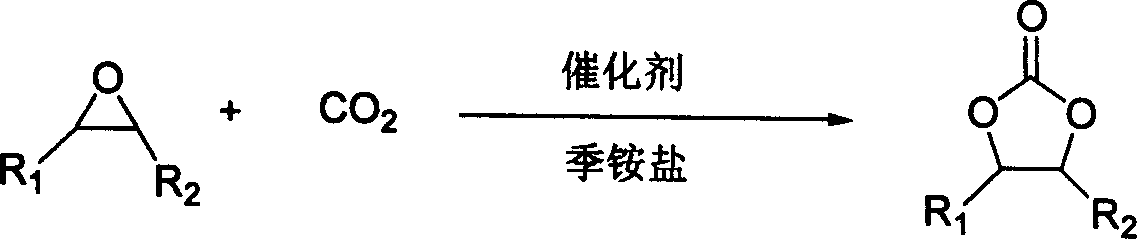
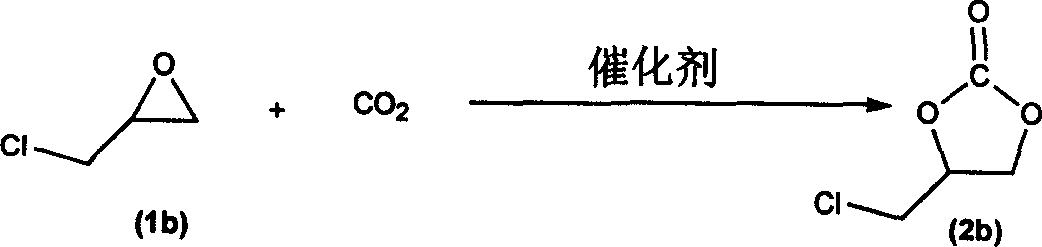
Process for synthesizing cyclic carbonic ester
A technology of cyclic carbonate and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of difficult separation of products and catalysts, high cost of catalysts, harsh conditions, etc., and achieve good industrial application prospects, simple catalyst system, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] In a 100ml autoclave, add 0.05mmol of zinc chloride, 0.9mmol of 1-methyl-3-butylimidazolium bromide successively, and finally add 20ml of propylene oxide (1a), close the reaction vessel, and fill a small amount of Measure the pressure of carbon dioxide, slowly raise the temperature to 100°C by the temperature controller, and then rise to the desired pressure, react for 1 hour, cool to room temperature, unload the kettle, and decompress the liquid obtained from the reaction. The raw material, then obtain the corresponding cyclic carbonate, weigh, calculate the yield and conversion frequency. After qualitative and quantitative analysis by 6890 / 5973 chromatography-mass spectrometry and NMR, the purity of the product is greater than 99%, and the separation yield is preferably 99%. The liquid obtained by the reaction is distilled under reduced pressure to obtain propylene carbonate (2a). The selectivity is 99%, and the conversion frequency (TOF) is 5410h -1 .
Embodiment 2
[0026] Same as Example 1, the binary catalyst system used is zinc bromide and 1-methyl-3-butyl imidazolium bromide to obtain propylene carbonate (2a). The selectivity is 99%, and the conversion frequency (TOF) is 5467h -1 .
Embodiment 3
[0028] Same as in Example 1, the binary catalyst system used is zinc iodide and 1-methyl-3-butylimidazolium bromide to obtain propylene carbonate (2a). The selectivity is 99%, and the conversion frequency (TOF) is 5467h -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com