Liquid crystal epoxy resin with branched chain, its preparing method, composition and use
A composition and compound technology, applied in liquid crystal materials, chemical instruments and methods, other chemical processes, etc., can solve the problems of insufficient adjustment of molecular chains, difficult to control curing reaction, high melting point, high shape memory recovery temperature, avoidance of High temperature curing defects, low deformation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
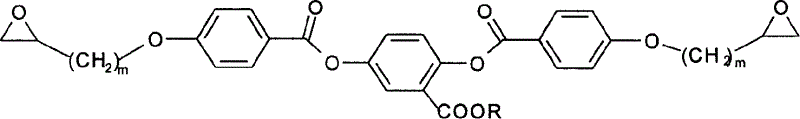
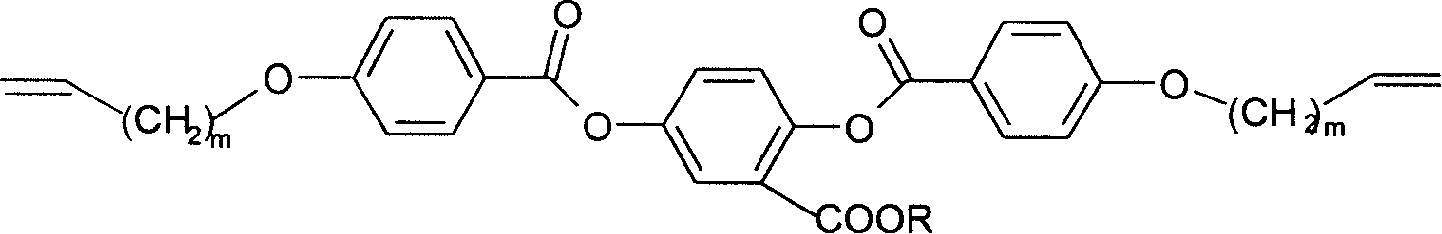
[0034]Add 0.01mol allyl chloride, 0.01mol methyl p-hydroxybenzoate, 1g 18-crown-6-ether and 10g potassium carbonate to 100mL acetone, and react at 50°C for 20 hours to obtain p-benzoic acid allyl ether. 0.01mol of 2,5-dihydroxybenzoic acid and 0.01mol of n-octanol were reacted at 80°C for 7 hours under the action of 6mL of concentrated sulfuric acid, extracted with 1000mL of solvent, the volume ratio of ethanol and water in the solvent was 3:1, In 2,5-dihydroxybenzoic acid n-octyl. 0.01mol p-benzoic acid allyl ether was acylated with 20mL thionyl chloride at 90°C, and the obtained acylated product was mixed with 0.01mol n-octyl 2,5-dihydroxybenzoate in 0.4g N,N-dimethyl Under the action of aminopyridine, react at 0°C to get m=1, R=C 8 h 17 The compound of formula (4). 0.01mol of this compound was reacted with 0.03mol of o-chloroperoxybenzoic acid at 45°C for 120 hours with 100mL of dichloromethane as a solvent, and the obtained product was confirmed to be R=C by nuclear mag...
Embodiment 2
[0037] Add 0.01mol 4-chloro-1-butene, 0.01mol methyl p-hydroxybenzoate, 1.5g dibenzo-18-crown-6-ether, 10g potassium carbonate to 150mL acetone and react at 65°C for 20 hours to obtain Butyl p-benzoate. 0.01mol of 2,5-dihydroxybenzoic acid and 0.01mol of n-tetradecyl alcohol were reacted at 120°C for 6 hours under the action of p-toluenesulfonic acid, extracted with 1000mL solvent, and the volume ratio of ethanol and water in the solvent was 5: 1. Obtain n-tetradecyl 2,5-dihydroxybenzoate. 0.01mol of butyl p-benzoate was acylated in thionyl chloride at 90°C, and the obtained acylated product was mixed with 0.01mol of n-tetradecyl 2,5-dihydroxybenzoate in 1g of N,N-di Under the action of methylaminopyridine, react at -5°C for 16 hours to obtain m=2, R=C 14 h 29 The compound of formula (4). 0.01mol of this compound was reacted with 0.03mol of o-chloroperoxybenzoic acid at 40°C for 150 hours with 100ml of dichloromethane as a solvent, and the obtained product was confirmed to...
Embodiment 3
[0040] Add 0.01mol allyl chloride, 0.01mol methyl p-hydroxybenzoate, 0.7g 15-crown-5-ether, 6g sodium hydroxide to 100mL deionized water, and react at 50°C for 20 hours to obtain allyl p-benzoate base ether. 0.01mol of 2,5-dihydroxybenzoic acid and 0.01mol of 1-bromo-n-butane were reacted in 100mL of triethylamine at 30°C for 6 hours, rinsed with 1000mL of solvent, the solvent was 5% aqueous sodium bicarbonate, and then vacuum-dried , to obtain n-butyl 2,5-dihydroxybenzoate. 0.01mol p-benzoic acid allyl ether and 20ml thionyl chloride were acylated at 85°C, and the obtained acylated product was mixed with 0.01mol 2,5-dihydroxybenzoic acid n-butyl ester in 1g catalyst N,N-dimethyl Under the action of aminopyridine, react at -5°C for 20 hours to obtain m=1, R=C 4 h 9 The compound of formula (4). 0.01mol of this compound was reacted with 0.03mol of o-chloroperoxybenzoic acid at 50°C for 36 hours with 100mL of dichloromethane as a solvent, and the obtained product was confirme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com