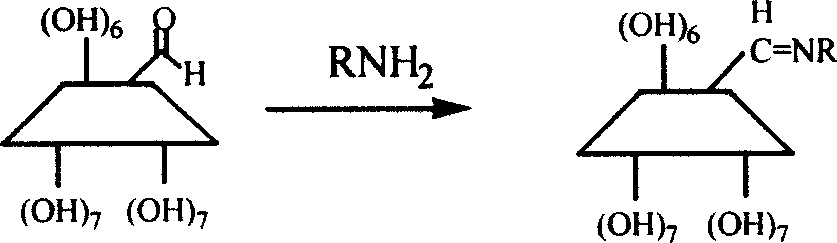
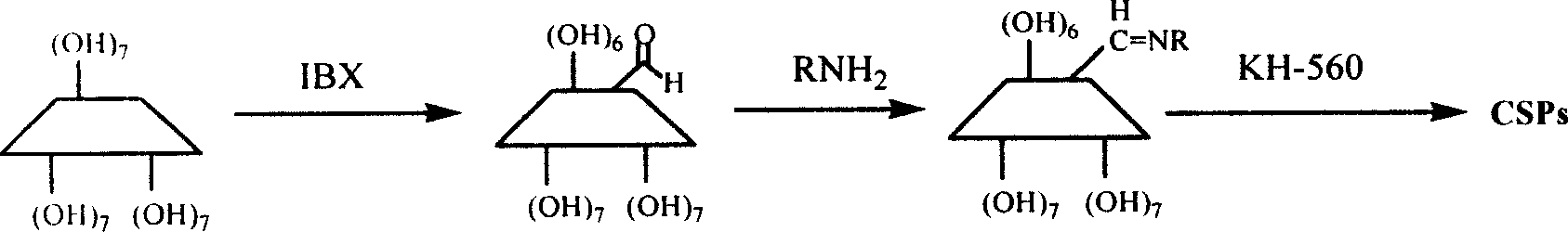Process for synthesizing alpha-Schiff base derivatized beta-cyclodextrin
A synthesis method and cyclodextrin technology are applied in the field of synthesis of α-Schiff base derivatized β-cyclodextrin bonded silica chiral stationary phase, which can solve the problem of chiral separation and identification of samples with new polymer chiral columns. The problems of single species and single chiral column species can achieve the effect of recovery and recycling, simple synthesis route and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 3g of cyclodextrin aldehyde and 45ml of pyridine to a 100ml reaction flask, turn on magnetic stirring, open the nitrogen protection valve, add 4.4ml of aniline solution, control the temperature of the external heating device to 25-30°C, stop heating and nitrogen protection after 4 days of reaction. The reaction liquid was dropped into acetone, cooled for 4 hours, and filtered with suction to obtain a solid which was washed twice with acetone to obtain a 6-aniline-β-cyclodextrin solid. The conversion rate of cyclodextrin aldehyde was 100%, and the yield of 6-aniline-β-cyclodextrin was 94%. Reaction conditions not mentioned in each comparative example and
[0018] Same as Example 1:
Embodiment 2
[0020] The conditions are the same as in Example 1, except that the addition of the aniline solution is changed to the addition of isopropylamine. The reaction result is: the conversion rate of cyclodextrin aldehyde is 100%, and the yield of 6-isopropylimine-β-cyclodextrin is 96%.
Embodiment 3
[0022] The conditions are the same as in Example 1, only the addition of the aniline solution is changed to the addition of R-(+)-phenylethylamine. The result of the reaction is: the conversion rate of cyclodextrin aldehyde is 100%, 6-R-(+)-phenylethyl The yield of β-cyclodextrin imine is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com