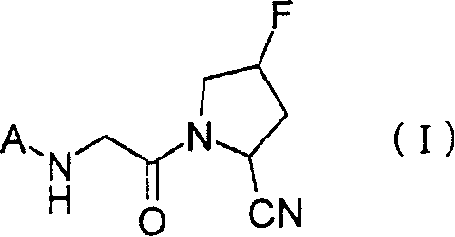
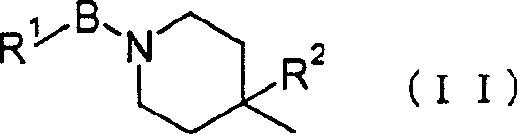
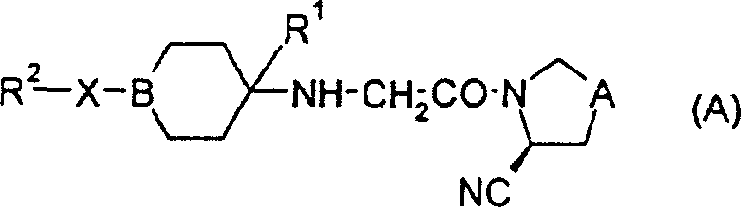
2-cyano-4-fluoropyrrolidine derivative or its salt
A fluoropyrrolidine and pyrrolidine technology, applied in the field of 2-cyano-4-fluoropyrrolidine derivatives or their salts, can solve the problem of weakening the effect of GLP-1 incretins and hindering the binding of active GLP-1, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0116] Into a solution of 0.73 ml of chloroacetyl chloride in 14 ml of chloroform under ice-cooling, 1.4 g of (2S, 4S)-4-fluoro A suspension of pyrrolidine-2-carboxamide monohydrochloride and 3.0 ml of N,N-diisopropylethylamine in 10 ml of chloroform was stirred for 30 minutes under ice-cooling. The reaction mixture was concentrated under reduced pressure, 2.4 ml of trifluoroacetic anhydride was added dropwise to a solution of the obtained residue in 14 ml of chloroform under ice-cooling, and the reaction mixture was warmed to room temperature and stirred for 1 hour. The reaction mixture was concentrated under reduced pressure, 0.1M aqueous hydrochloric acid solution was added to the obtained residue, extraction was performed with ethyl acetate, and the organic layer was dried over anhydrous magnesium sulfate. The desiccant was removed, the solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (developing s...
reference example 5
[0120] According to J.Med.Chem (1991), 34,656-663, J.Heterocycl.Chem.(1982), 790mg of synthesis of the method described in 485-488 outside -8-azabicyclo[3.2.1] To a suspension of tert-butyl oct-3-ylcarbamate hydrochloride in 10ml of dichloromethane and 5ml of N,N-dimethylformamide, add 1.3g of triethylamine and 1.05g of methanesulfonyl chloride, at room temperature Stir for 1 day. The reaction mixture was concentrated under reduced pressure, and water was added to the residue, followed by extraction with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography (developing solvent: hexane: ethyl acetate = 7:3), and crystallized from diethyl ether-hexane to obtain 600 mg of exo-8-(methylsulfonyl)-8 - tert-butyl azabicyclo[3.2.1]oct-3-ylcarbamate Colorless solid.
[0121] Reference Examples 6 to 16 sho...
reference example 17
[0123] To a mixed solution of 4.7 ml of acetic anhydride and 1.9 ml of formic acid was added a chloroform solution of 2.0 g of tert-butyl piperidin-4-ylcarbamate, and stirred at room temperature for 15 hours. Water was added to the reaction mixture, extracted with EtOAc, and the organic layer was washed with 1M aqueous hydrochloric acid, saturated aqueous sodium bicarbonate and saturated brine. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography (developing solvent: chloroform:MeOH=50:1) to obtain 1.7 g of tert-butyl (1-formylpiperidin-4-yl)carbamate.
[0124] Reference Example 18 shown in Table 4 was produced in the same manner as Reference Example 17 using its corresponding raw materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com