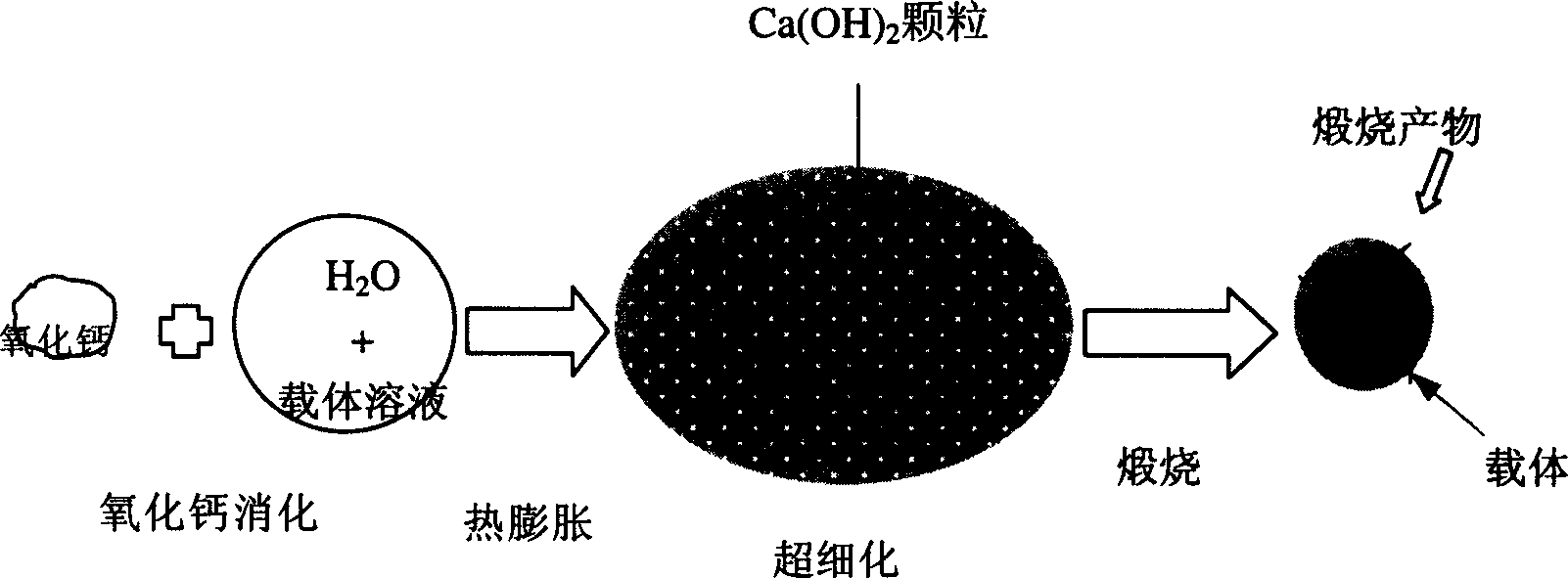
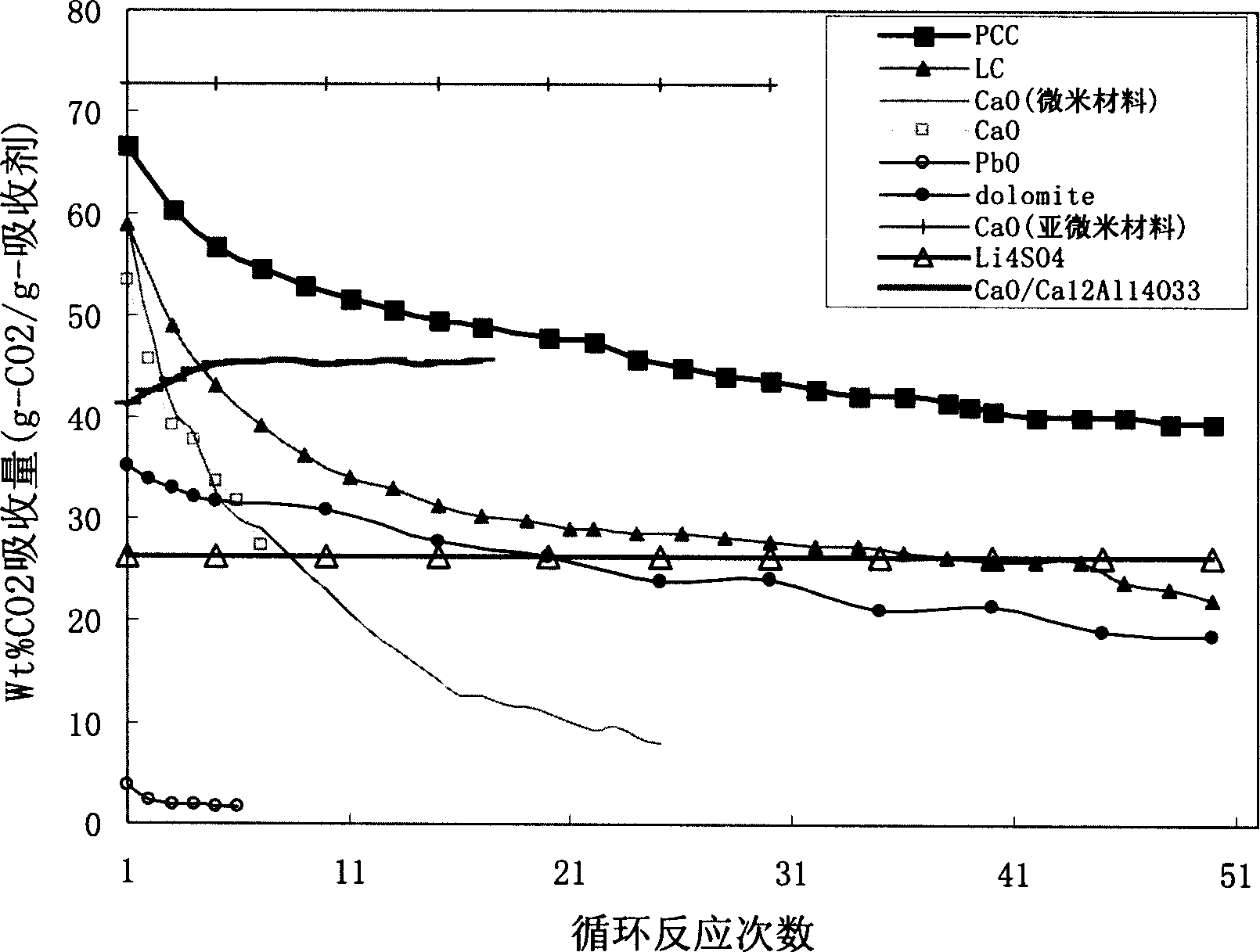
High active calcium-base CO2 absorbent and its preparing method
An absorbent and high-activity technology, which is applied in separation methods, chemical instruments and methods, and other chemical processes, can solve problems such as activity decline, carbon dioxide absorption capacity reduction, and obstacles to large-scale application of technology, and achieve stable and high reactivity. The effect of carbon dioxide absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation raw materials: 2-propanol, distilled water, aluminum nitrate 9 water, CaO
[0028] Preparation steps:
[0029] (1) calcining calcium oxide at 900°C for 2 hours;
[0030] (2 Add weighed calcined calcium oxide at 25°C, then add isopropanol, then add distilled water;
[0031] (3) adding aluminum nitrate 9 water;
[0032] (4) stirring in a constant temperature water bath at 75°C for 1 hour;
[0033] (5) Dry at 100°C until the moisture in the sample evaporates, and record the drying time;
[0034] (6) Take out the dried sample, calcinate it at 500° C. for 3 hours, then take it out, and grind the sample finely.
[0035] (7) Add distilled water and dry at 80°C for 2 hours;
[0036] (8) calcining the dried sample at 900°C for 1.5 hours;
[0037] (9) Grind the sample finely to make highly active calcium-based CO 2 absorbent.
[0038] CaO / Ca 12 Al 14 o 33 CaO(g)Al(NO 3 ) 3 .9H 2 O (g) distilled water (ml) (CH 3 ) 2 CHOH (mol)
Embodiment 2
[0040] Preparation raw materials: ethanol, distilled water, anhydrous aluminum chloride, calcium oxide
[0041] Preparation steps:
[0042] (1) calcining calcium oxide at 800°C for 4 hours;
[0043] (2) add weighed calcined calcium oxide at room temperature, then add ethanol, then add distilled water;
[0044] (3) adding aluminum salt;
[0045] (4) stirring in a constant temperature water bath at 75°C for 1 hour;
[0046] (5) Dry at 100°C until the moisture in the sample evaporates;
[0047] (6) Take out the dried sample, calcinate it at 600°C for 3 hours, then take it out, grind the sample finely;
[0048] (7) Add distilled water and dry at 80°C for 2 hours;
[0049] (8) Calcining the dried sample at 700° C. for 3 hours.
[0050] (9) Grind the sample finely to make highly active calcium-based CO 2 absorbent.
[0051] CaO / Ca 12 Al 14 o 33 CaO(g)AlCl 3 (g) distilled water (ml) CH 3 CH 2 OH (mol)
Embodiment 3
[0053] Preparation raw materials: methanol, distilled water, aluminum nitrate 9 water, CaO
[0054] Preparation steps:
[0055] (1) calcining calcium oxide at 1000°C for 1 hour;
[0056] (2) Add weighed calcined calcium oxide at 25°C, then add methanol, then add distilled water;
[0057] (3) adding aluminum nitrate 9 water;
[0058] (4) stirring in a constant temperature water bath at 75°C for 1 hour;
[0059] (5) Dry at 100°C until the moisture in the sample evaporates, and record the drying time;
[0060] (6) Take out the dried sample, calcinate it at 700°C for 3 hours, then take it out, grind the sample finely;
[0061] (7) Add distilled water and dry at 80°C for 2 hours;
[0062] (8) calcining the dried sample at 1100° C. for 1 hour;
[0063] (9) Grind the sample finely to make highly active calcium-based CO 2 absorbent.
[0064] CaO / Ca 12 Al 14 o 33 CaO(g)Al(NO 3 ) 3 .9H 2 O (g) distilled water (ml) CH 3 OH (mol)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com