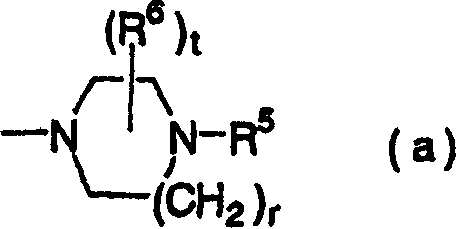Spiro compounds, medicinal compositions containing the same and intermediates of the compounds
A technology of spiro compounds and compounds, applied in the field of new spiro compounds, can solve the problems of no SERM usefulness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
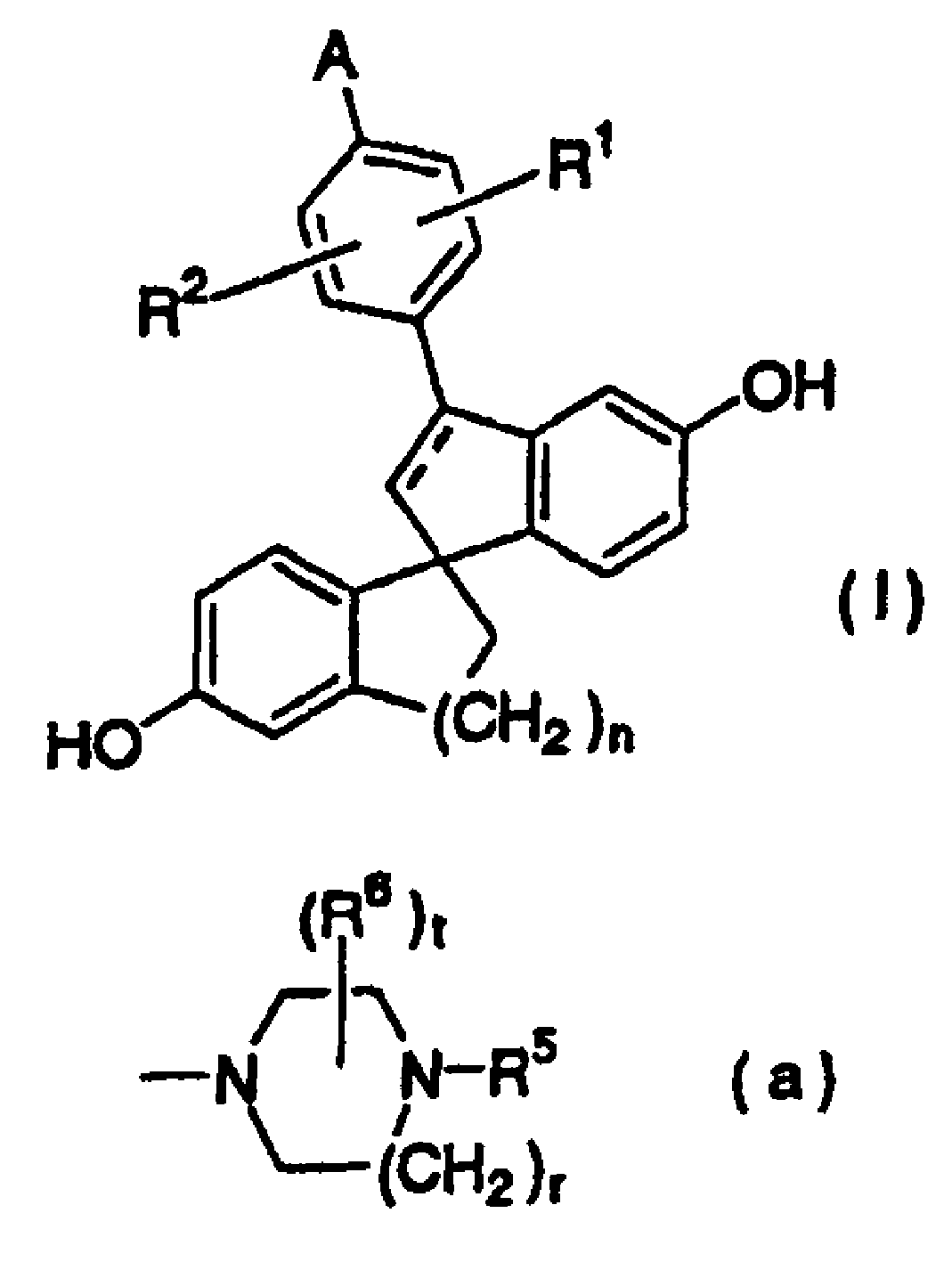
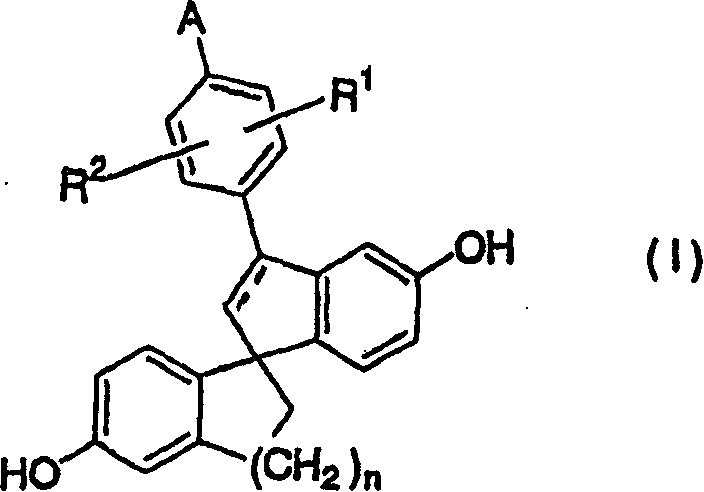
[0308] Preparation of 5-methoxy-2-(5-methoxy-1-indanyl)benzoic acid:
[0309] (1) Suspend 22.3g of magnesium in 400mL of tetrahydrofuran, add 243g of 2-(2-bromo-5-methoxyphenyl)-4,4-dimethyl-4,5-dihydrooxazole to it 1 / 10 of the tetrahydrofuran (2L) solution. A small amount of iodine was added, and the reaction solution was heated to reflux. After the violent reaction was over, the remaining oxazole solution was added thereto under heating and reflux in 1 hour, and then heated and refluxed for 2 hours after the dropwise addition. Thereto was added dropwise a solution of 118 g of 5-methoxy-1-indanone in tetrahydrofuran (1 L) over 0.5 hours under ice-cooling. After stirring at room temperature for a whole day and night, 400 mL of saturated ammonium chloride aqueous solution was added and stirred. After separating the organic phase, the organic phase was washed with water and saturated brine. The organic phase was dried over sodium sulfate and concentrated under reduced pressu...
reference example 2
[0314] Preparation of ethyl 2-diazo-3-[5-methoxy-2-(5-methoxy-1-indanyl)phenyl]-3-oxopropionate:
[0315] (1) Suspend 83g of 5-methoxy-2-(5-methoxy-1-indanyl)benzoic acid in 300mL of toluene, add 33.2mL of oxalyl chloride and 0.1mL of dimethylformamide, at room temperature Stirring was continued for 4 hours. The reaction solution was concentrated under reduced pressure to obtain an oily substance.
[0316] (2) Dilute 789 mL of 20% hexamethyldisilazane potassium salt toluene solution with 700 mL of tetrahydrofuran, add 68 mL of ethyl acetate dropwise thereto at -78°C over 1 hour, and then stir at the same temperature for 1 hour. A solution of the above oil in tetrahydrofuran (300 mL) was added dropwise thereto over 1 hour, followed by stirring for another 1 hour. 600 mL of saturated ammonium chloride aqueous solution was added to terminate the reaction. After separating the organic phase, the organic phase was washed with water and saturated brine. After drying the organic p...
reference example 3
[0320] Preparation of 1-(4-bromophenyl)-4-ethylpiperazine
[0321] (1) Add 20 mL of water and 0.6 g of sodium bicarbonate to a solution of 1.0 g of 1-(4-bromophenyl)piperazine in ethyl acetate (20 mL), and stir vigorously. 0.5 mL of acetic anhydride was added thereto, and stirring was continued at room temperature for 2 hours. The organic phase was separated, washed successively with saturated aqueous sodium bicarbonate solution and saturated brine, and dried over sodium sulfate. The organic phase was concentrated under reduced pressure to give an oil.
[0322] (2) The above oil was dissolved in 5 mL of tetrahydrofuran, 4.5 mL of 2.0 M borane dimethyl sulfide complex in tetrahydrofuran was added, and stirred at room temperature for a whole day and night. After adding 10 mL of 20% hydrochloric acid and heating to reflux for 1 hour, tetrahydrofuran was distilled off under reduced pressure. A 15% aqueous sodium hydroxide solution was added until the pH of the reaction liquid r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com