Nanometer alpha-nickel hydroxide and its prepn
A nickel hydroxide and potassium hydroxide technology, applied in nickel oxide/nickel hydroxide and other directions, can solve the problems of reducing electrode discharge specific capacity, low electrode discharge capacity, complicated process, etc., and achieves improved proton conductivity and electrochemical performance. The effect of improving, high discharge specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
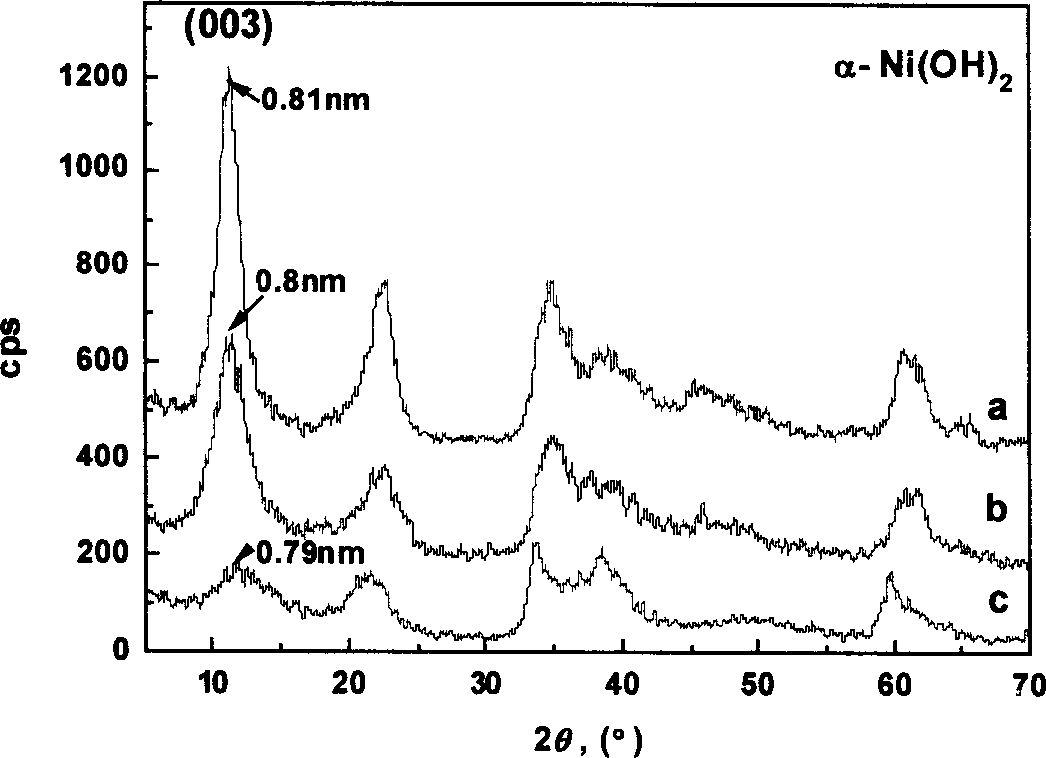
Image
Examples
Embodiment 1
[0031] The first step takes 1.0 moles of nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 0.05 moles (equivalent to 5% of the moles of nickel salt) of aluminum nitrate (Al(NO 3 ) 3 9H 2 0) dissolve with natural water, be mixed with the reaction solution that 100ml nickel ion concentration is 10mol / L;
[0032] The potassium hydroxide of second step 1.2 moles is dissolved in 100ml natural water and is mixed with the concentration of potassium hydroxide and is the precipitation agent solution of 12mol / L;
[0033] The third step is to add the precipitant solution dropwise to the reaction solution under the condition of stirring with the mixer and dispersing with ultrasonic waves at the same time, control the reaction temperature at 20°C, control the reaction pH to about 7.0, and the dropwise reaction time is about 15 minutes. After the addition, stop stirring and continue ultrasonic dispersion for 300 minutes to obtain a green or blue-green nickel hydroxide precipitation product;
...
Embodiment 2
[0037] The first step takes 0.05 mole of nickel sulfate (NiSO 4 ·6H 2 O), 0.0025 moles (equivalent to 5% of the moles of nickel salt) iron nitrate (Fe(NO 3 ) 3 ·7H 2 O) and 0.0025 moles (equivalent to 5% of the moles of nickel salt) aluminum chloride (AlCl 3 ), dissolved in distilled water to prepare 5000ml of nickel ion concentration is the reaction solution of 0.01mol / L;
[0038] The second step takes 0.15 moles of potassium hydroxide (equivalent to 300% of the number of moles of nickel ions) and dissolves it in distilled water to prepare 3000ml of potassium hydroxide concentration as an oxidant solution of 0.05mol / L;
[0039] The third step is to add the precipitant solution dropwise to the reaction solution under the condition of stirring with the mixer and dispersing with ultrasonic waves at the same time, control the reaction temperature at 30°C, the reaction pH is about 9.5, the dropwise reaction time is 120 minutes, and the dropwise addition is completed Finally, ...
Embodiment 3
[0043] The first step takes 0.5 mole of nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O), 0.065 moles (equivalent to 13% of the moles of nickel salt) of aluminum nitrate (Al(NO 3 ) 3 9H 2 (2) is dissolved in distilled water and is mixed with the reaction solution that 2000ml nickel ion concentration is 0.25mol / L;
[0044] The second step takes 1.1 moles (equivalent to 220% of the number of moles of nickel ions) potassium hydroxide, and is mixed with distilled water to make oxidant solution 275ml of potassium hydroxide concentration about 4mol / L;
[0045] The third step is to add the precipitant solution to the reaction solution drop by drop under the condition of stirring with the mixer and dispersing with ultrasonic wave at the same time. Control the reaction temperature at 35°C and control the reaction pH to about 8.0. After the end, stop stirring and continue ultrasonic dispersion for 60 minutes to obtain a green or blue-green nickel hydroxide precipitation product;
[0046] The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com