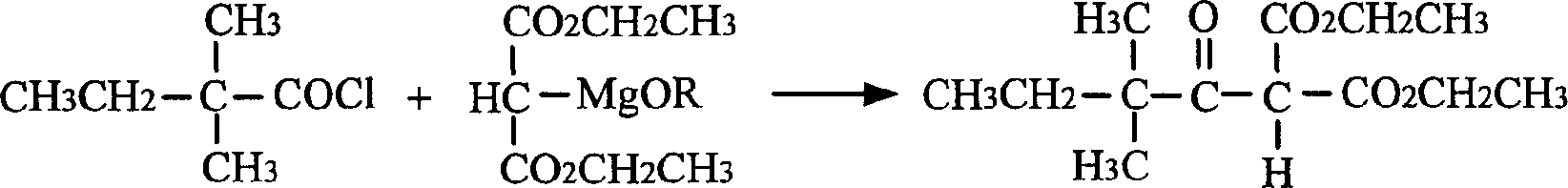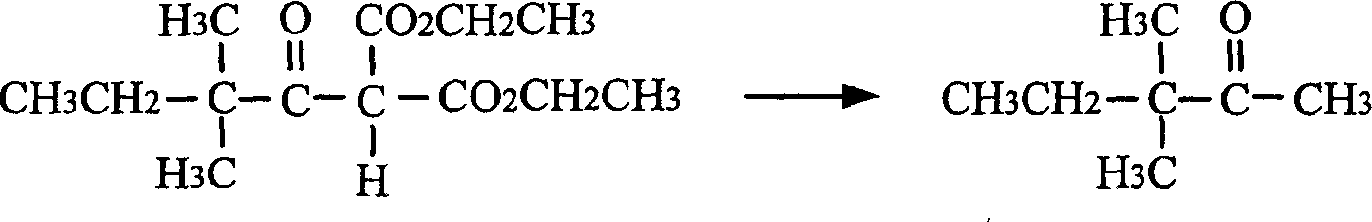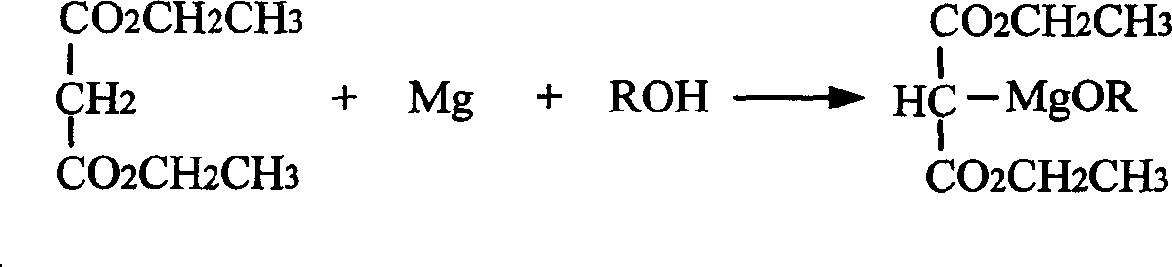Process for preparing 3, 3-dimethyl -2-pentanone
A technology for the reaction of dimethyl and dimethyl butyryl chloride is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of poor safety, difficult separation of by-products and products close to boiling points, and expensive raw materials. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] Raw material preparation
[0023] (1) Preparation of 2,2-dimethylbutyryl chloride
[0024] Under stirring, add 25.6g of 2,2-dimethylbutyric acid and 35.8g of thionyl chloride into the three-necked flask, heat to reflux, and absorb the generated hydrogen chloride and sulfur dioxide gas with lye. After no gas was produced, excess thionyl chloride was distilled off, and then distilled under reduced pressure to obtain 26.9 g of 2,2-dimethylbutyryl chloride, with a yield of 90%.
[0025] (2) Preparation of Diethyl Malonate Ethoxymagnesium
[0026] Under stirring, add 4g of absolute ethanol, 5.4g of magnesium powder, and then 0.5mL of carbon tetrachloride into the three-necked flask, and start the reaction after 3-5 minutes, and heat it slightly if necessary. After the reaction moderated, 150 mL of benzene was slowly added, and the reaction was kept boiling. Under stirring, a mixed solution of 35.2 g of diethyl malonate, 20 mL of absolute ethanol and 25 mL of benzene was ...
Embodiment 1
[0028] (1) 2, the preparation of 2-dimethyl butyryl malonate diethyl ester
[0029] Under stirring, a mixed solution of 26.9g of 2,2-dimethylbutyryl chloride and 50mL of benzene was added dropwise to the diethyl malonate ethoxymagnesium solution prepared above, and the dropwise addition was completed after about 15 minutes. Continue to stir and heat to reflux for about 1h. After the reaction solution was cooled, 220 mL of 10% sulfuric acid was added for acidification. The organic layer was separated, the aqueous layer was extracted once with 75 mL of benzene, and combined with the organic layer. The organic layer was washed with water, and benzene was recovered by distillation to obtain diethyl 2,2-dimethylbutyrylmalonate, which was used in the next reaction.
[0030] (2) Preparation of 3,3-dimethyl-2-pentanone
[0031] Under stirring, add 40mL of water, 14g of concentrated sulfuric acid, 60mL of acetic acid and the diethyl 2,2-dimethylbutyrylmalonate obtained from the abov...
Embodiment 2
[0035] Using tetrahydrofuran instead of benzene as the solvent, the preparation of the starting material and the step (1) of Example 1 were repeated. The reaction conditions of the step (2) of Example 2 are the same as those of the step (2) of Example 1, and finally 3,3-dimethyl-2-pentanone is obtained with a content of 98.6% and a yield of 81%. The resulting product is determined by infrared spectroscopy and mass spectrometry, and the results are the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com