Tech. for synthetic (4-chlorobutyl) 1-cyclohexyl-1,2,3,4-tetrazole
A production process, cyclohexyl technology, applied in the direction of organic chemistry, can solve the problems of hydrogen chloride pollution, high price, prone to explosion production accidents, etc., and achieve the effect of not being prone to accidents, not easy to decompose or volatilize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
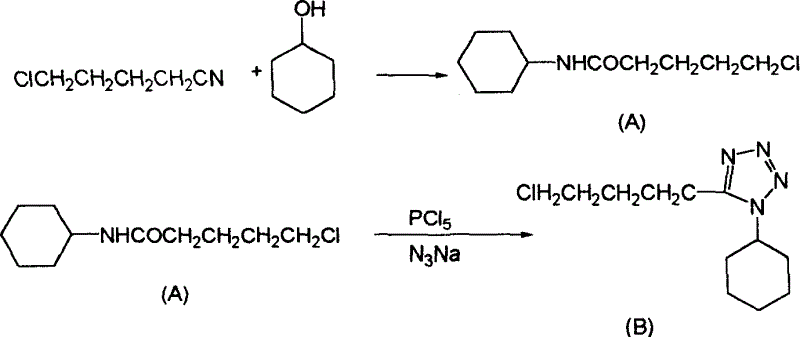
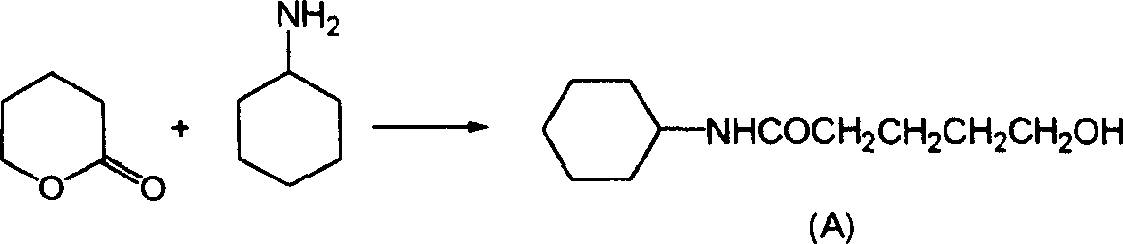
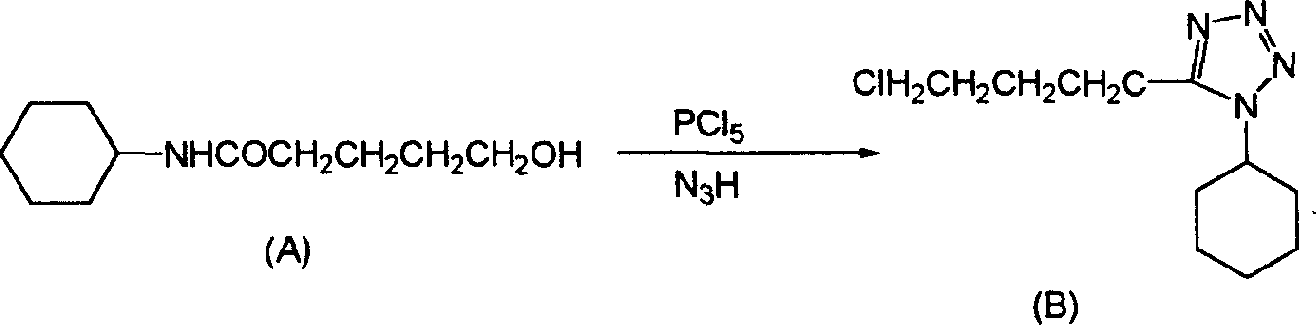
[0019] The synthetic production process of this finished product is illustrated as follows:
[0020] first step:
[0021] 200 kilograms of 5-chlorovaleronitrile and 210 kilograms of cyclohexanols are dropped into the first reactor of 500 liters, and 50 kilograms of concentrated sulfuric acid of 98% concentration are dropped into the first reactor, and the temperature is raised to 60 DEG C from room temperature, and the reaction is kept for 15 After the reaction was completed, the temperature was cooled to room temperature for 1 hour to obtain a 5-chloro-N-cyclohexylpentanamide reaction solution. Add 500 kg of water in advance to the 1000 liter second reaction kettle, ice brine in the jacket, and cool the second reaction kettle to 0°C.
[0022] Slowly pump the cooled 5-chloro-N-cyclohexylpentanamide reaction solution in the 500-liter first reaction kettle into the above-mentioned 1000-liter second reaction kettle under a negative pressure of 500-600 mm Hg, and draw the materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com