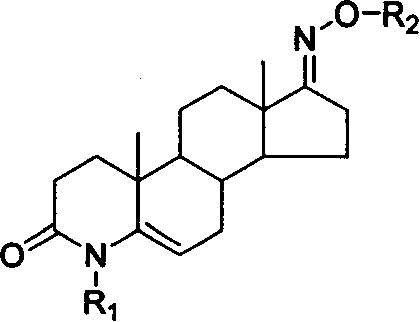
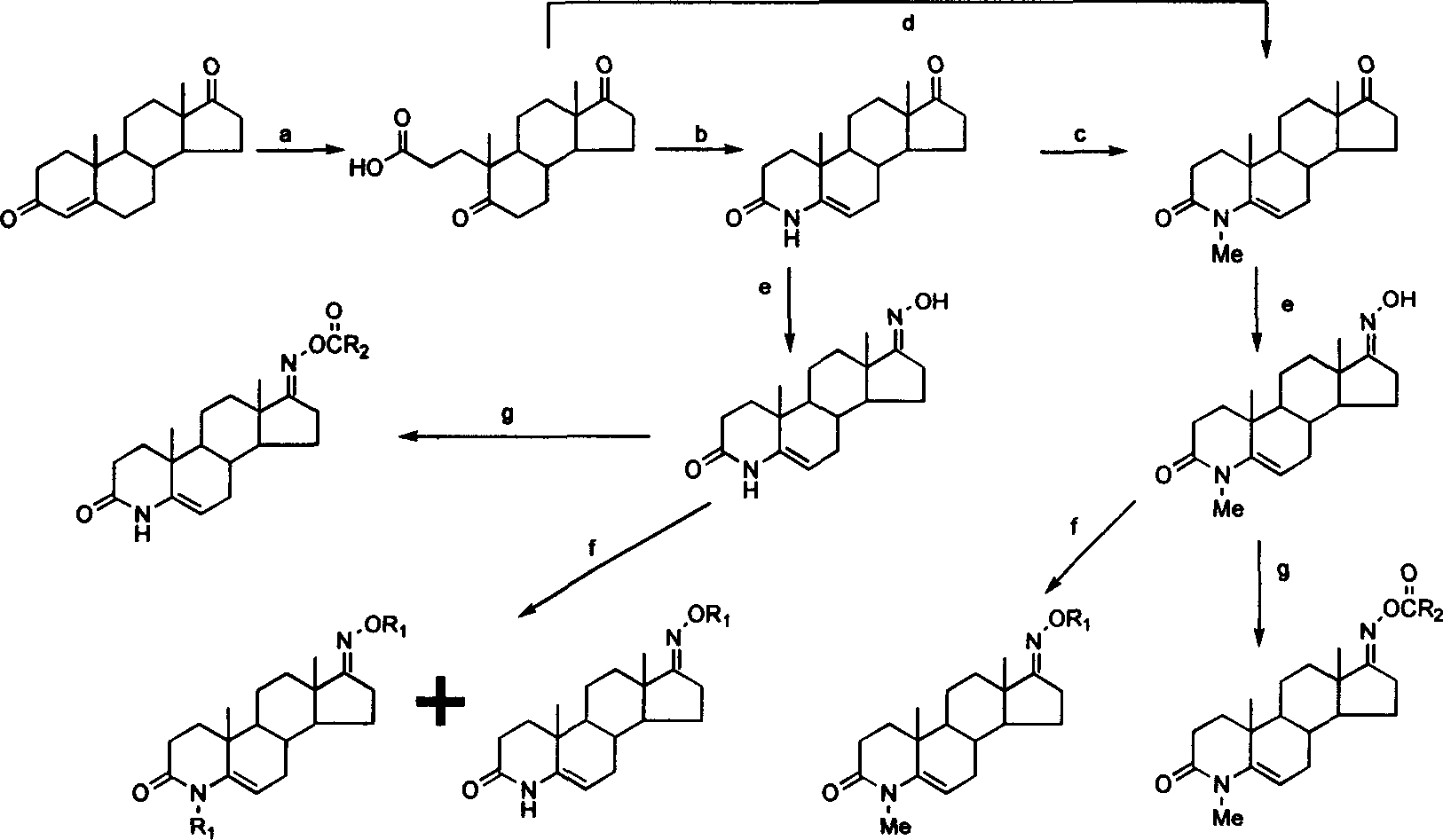
Steroid compound with 5-alpha reductase active and preparation process thereof
A technology of steroidal compounds and inhibitory activity, which is applied in the field of medicinal chemistry and can solve the problems of reproductive system toxic side effects and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: 3-carbonyl-4-ethyl-4-aza-5-androstene-17-oxime N-O-ethyl ether
[0025] Step 1: Preparation of 5,17-carbonyl-A-anorca-3,5-cleavage-androstane-3-carboxylic acid:
[0026] Dissolve androst-4-ene-3,17-dione (10.0g, 35.0mmol) in tert-butanol (350mL), add anhydrous sodium carbonate solution (23g, 20%) to it under stirring, and raise the temperature To 90°C, slowly add a solution of sodium periodate (41.2g, 0.2mol) and potassium permanganate (0.33g, 2.1mmol) dissolved in water (250mL) dropwise, and the dropwise is completed within about 1.5h. After the dropwise addition, reflux for 5h. Cool to room temperature, filter, wash the filter cake with water (50mL×4), distill off most of the tert-butanol from the filtrate under reduced pressure, adjust the pH to ≈1.5 with dilute hydrochloric acid, extract with dichloromethane (100mL×3), and dry over anhydrous sodium sulfate , filtered, and concentrated under reduced pressure to obtain a light yellow paste. Column chroma...
Embodiment 2
[0033] Example 2: Preparation of 3-carbonyl-4-benzyl-4-aza-5-androstene-17-oxime N-O-benzyl ether:
[0034] Step 1, step 2, and step 3 of this embodiment are the same as in embodiment 1.
[0035] Step 4: Preparation of 3-carbonyl-4-benzyl-4-aza-5-androstene-17-oxime N-O-benzyl ether:
[0036] 3-Carbonyl-4-aza-5-androstene-17-oxime (0.5g, 1.66mmol) and potassium hydroxide (0.38g, 6.64mmol) were added to N,N-dimethylformamide (60mL ), stirred and dissolved, added dropwise benzyl chloride (1.2mL, 9.93mmol), and reacted at 70°C for 12h. The reaction solution was cooled to room temperature, added with water (100 mL), and extracted with dichloromethane (50 mL×3). The organic phase was washed with distilled water (50 mL×10), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated by column chromatography (methanol / dichloromethane=1 / 99) to obtain 0.46 g of a white solid. 1 H NMR (400MHz, CDCl 3 )δ: 0.93(s, 3H, 18-CH 3 ), 1.08(s, 3H, 19-CH...
Embodiment 3
[0037] Example 3: Preparation of 3-carbonyl-4-isobutyl-4-aza-5-androstene-17-oxime N-O-isobutyl ether:
[0038] Step 1, step 2, and step 3 of this embodiment are the same as in embodiment 1.
[0039] Step 4: Preparation of 3-carbonyl-4-isobutyl-4-aza-5-androstene-17-oxime N-O-isobutyl ether:
[0040] 3-Carbonyl-4-aza-5-androstene-17-oxime (0.5g, 1.66mmol) and potassium hydroxide (0.23g, 4.1mmol) were added to N,N-dimethylformamide (50mL ), stirred and dissolved, added isoiodobutane (1.52mL, 9.93mmol) dropwise, and reacted at 30°C for 20h. The reaction solution was cooled to room temperature, added with water (100 mL), and extracted with dichloromethane (50 mL×3). The organic phase was washed with distilled water (50 mL×10), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated by column chromatography (methanol / dichloromethane=1 / 99) to obtain 0.04 g of a white solid. 1 H NMR (400MHz, CDCl 3 )δ: 0.88(s, 3H, 18-CH 3 ), 0.90 (s, 3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com