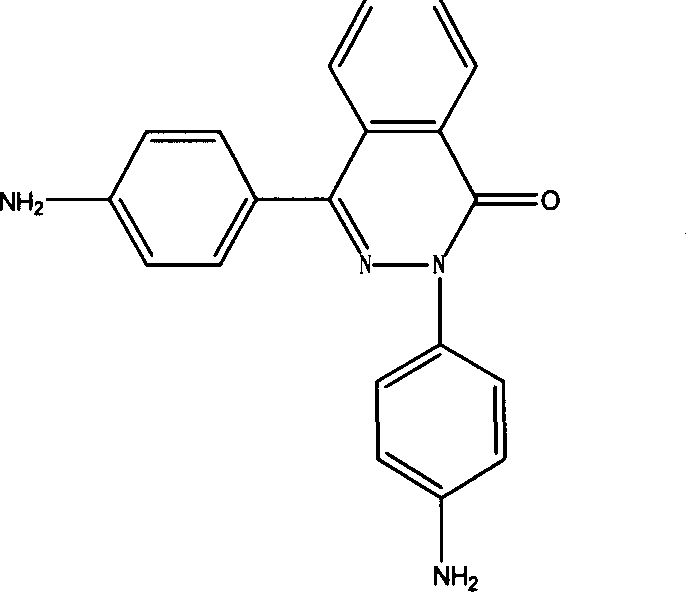
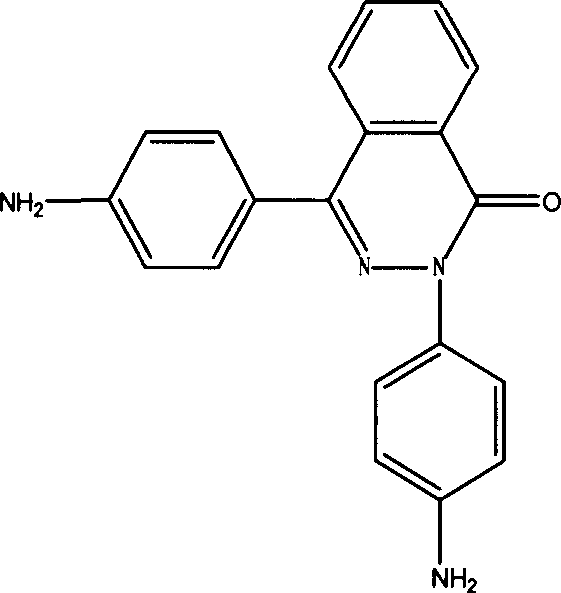
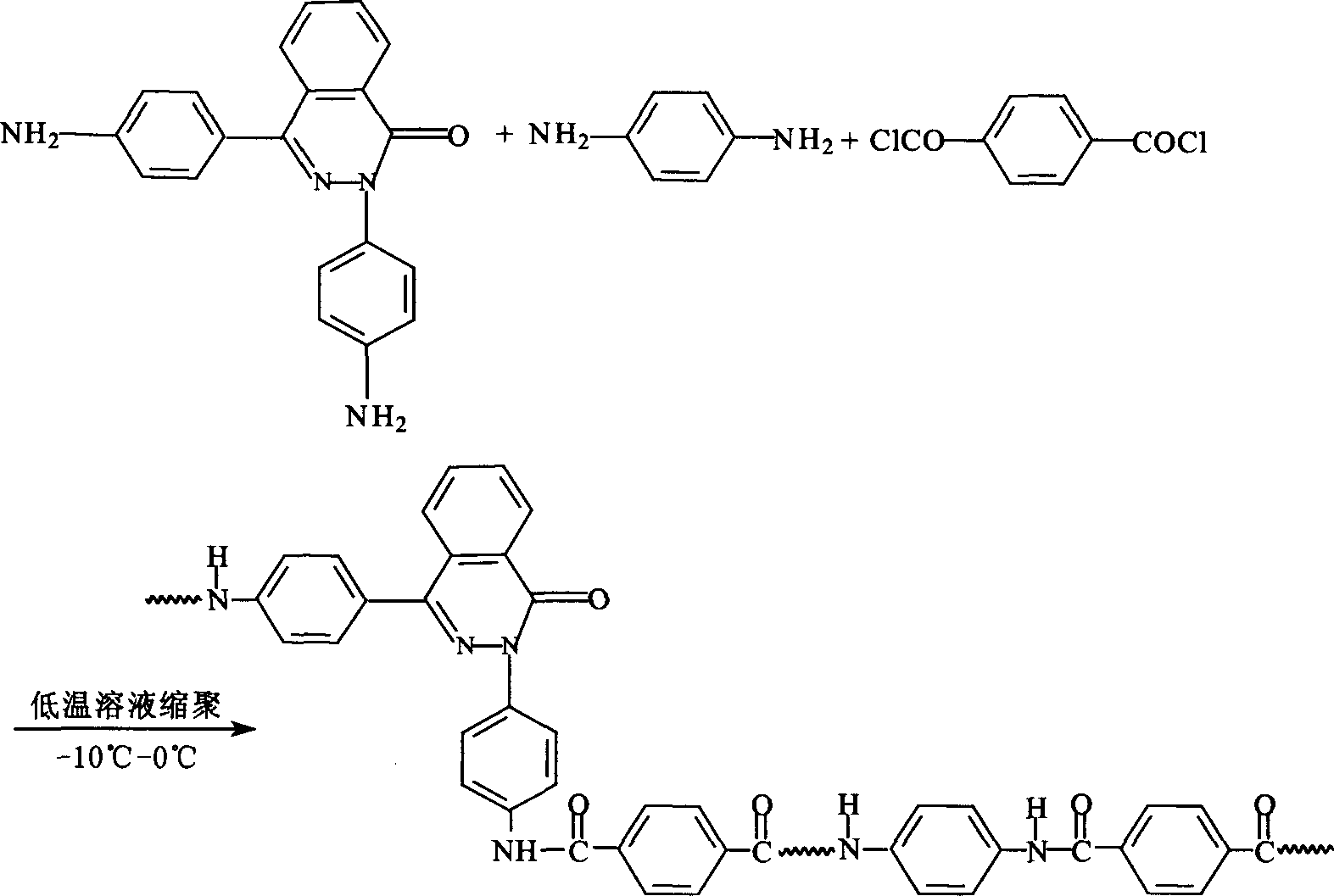
PPTA containing 2,4 di (4-amino phenyl)-2,3-diaza naphthalene-1-one and preparation process thereof
A technology of naphthyridine and aminophenyl, which is applied in the synthesis of high polymers and the polymerization of para-polyarylamides. It can solve the problems of insoluble and infusible PPTA resins, resistance to strong acid corrosion, and difficult processing, and achieve good results. Dissolution performance, good electrical insulation performance, simple and easy synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 100ml of NMP to a 250ml three-necked flask, N 2 Protect, stir, add a certain amount of CaCl 2 , slowly heat up to 70 ° C, after complete dissolution, cool to room temperature, add 3.078 g of p-phenylenediamine and 0.492 g of 2,4-bis(4-aminophenyl)-2,3-naphthalene-1-one , when all the materials were dissolved, cooled to -5°C, added 6.090 grams of terephthaloyl chloride, accelerated stirring until the phenomenon of climbing rods appeared, reacted for 30 minutes, and added 2.22 grams of Ca(OH) 2 Neutralize for 20 min to obtain a copolycondensation PPTA solution. The prepared solution is thoroughly washed with water and dried to obtain a novel PPTA resin, whose characteristic viscosity measured in 98% concentrated sulfuric acid is 3.2. The PPTA can be dissolved in NMP, or solution-spun.
Embodiment 2
[0017] Add 100ml of NMP to a 250ml three-necked flask, N 2 Protect, stir, add a certain amount of CaCl 2 , slowly heat up to 70 ° C, dissolve, cool to room temperature, add 3.888 g of p-phenylenediamine, wait for the material to be completely dissolved, cool to -5 ° C, add 8.12 g of terephthaloyl chloride, react for 10 min, and synthesize a certain degree of polymerization. Oligomer, add 1.312 g of 2,4-bis(4-aminophenyl)-2,3-naphthalene-1-one, after dissolving, add g terephthaloyl chloride, and accelerate stirring until there is climbing Phenomenon, react for 30min, add 2.96g Ca(OH) 2 Neutralize for 20 min to obtain a PPTA copolycondensation solution. The prepared solution was thoroughly washed with water and dried, and the prepared solution was thoroughly washed with water and dried to obtain a novel PPTA resin. The characteristic viscosity measured in 98% concentrated sulfuric acid was 2.8. The PPTA can be dissolved in NMP, or solution-spun.
[0018] The structure of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com