Process for preparing lithium ferrous phosphate coated with carbon
A lithium iron phosphate and carbon-coated technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of cumbersome process, high processing cost, long reaction time, etc., and achieve simple process route and processing cost. Low, low material cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Weigh iron phosphate, lithium acetate and ethylene glycol, the mol ratio of iron phosphate and lithium acetate is 1: 1, the mol ratio of iron phosphate and ethylene glycol is 1: 1, add distilled water, make lithium acetate and ethylene glycol The diol was dissolved, and then stirred at 60° C. for 10 hours until evaporated to dryness to obtain a lithium iron phosphate precursor.
[0026] (2) Under nitrogen protection, the lithium iron phosphate precursor was treated at 650° C. for 5 hours to obtain lithium iron phosphate.
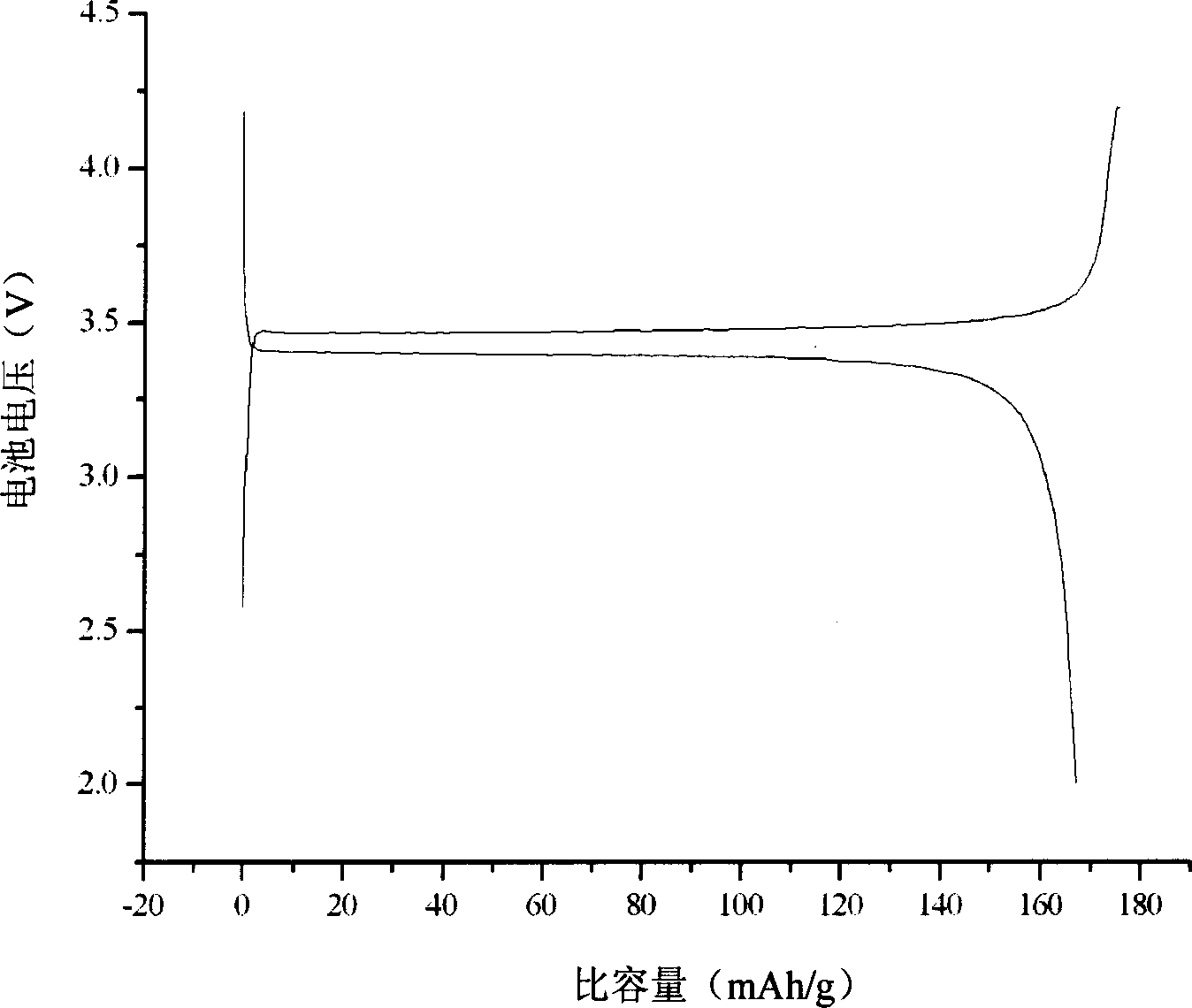
[0027] (3) Weigh lithium ferrous phosphate and sucrose, the mass ratio of the two is 95:5, dissolve the sucrose in distilled water, heat, stir and mix until evaporated to dryness. Then, under the protection of mixed gas of nitrogen and 5% hydrogen by volume, the process was carried out at 800° C. for 3 hours to obtain carbon-coated lithium iron phosphate.
Embodiment 2
[0029] (1) Weigh iron phosphate, lithium acetate and glycerin, the mol ratio of iron phosphate and lithium acetate is 1: 1, the mol ratio of iron phosphate and glycerin is 1: 2, add distilled water, make lithium acetate and glycerin The triol was dissolved, and then stirred at 70° C. for 8 hours until evaporated to dryness to obtain a lithium iron phosphate precursor.
[0030] (2) Under the protection of a mixed gas of argon and 5% hydrogen by volume, the lithium iron phosphate precursor was treated at 500° C. for 2 hours to obtain lithium iron phosphate.
[0031] (3) Weigh lithium ferrous phosphate and glucose, the mass ratio of the two is 90:10, dissolve the glucose in distilled water, heat and stir to mix until evaporated to dryness. Then, under the protection of a mixed gas of argon and 5% hydrogen by volume, it was treated at 700° C. for 4 hours to obtain carbon-coated lithium iron phosphate.
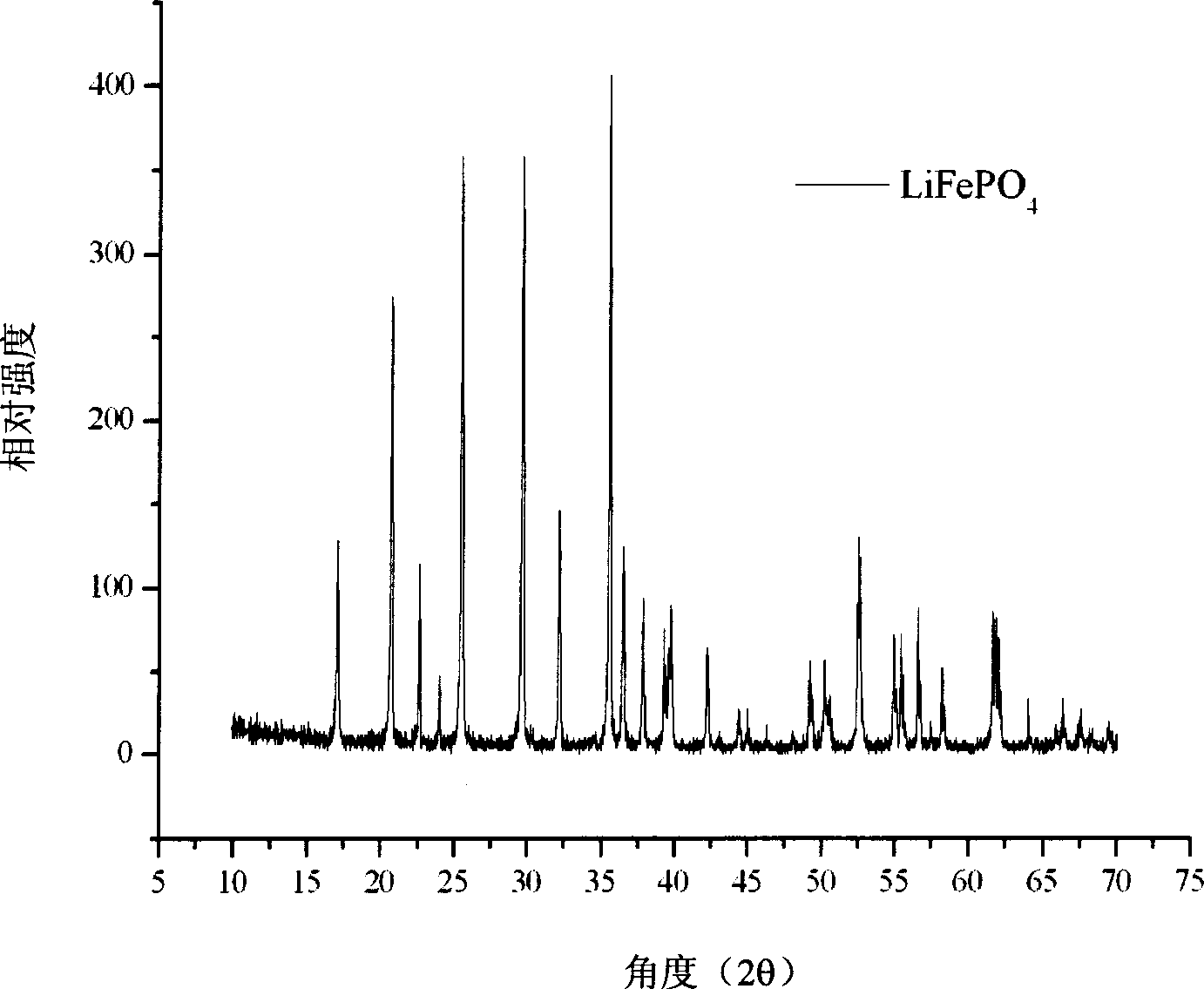
[0032] figure 1 is the XRD spectrum of carbon-coated lithium iron phosphate,...
Embodiment 3
[0034] (1) Weigh iron phosphate, lithium acetate and urea, the mol ratio of iron phosphate and lithium acetate is 1: 1, the mol ratio of iron phosphate and urea is 1: 3, add distilled water, lithium acetate and urea are dissolved, then in Stir at 80° C. for 6 hours until evaporated to dryness to obtain a lithium iron phosphate precursor.
[0035] (2) Under the protection of the mixed gas of argon and 5% hydrogen by volume, the lithium iron phosphate precursor was treated at 700° C. for 3 hours to obtain the lithium iron phosphate.
[0036] (3) Weigh lithium ferrous phosphate and sucrose, the mass ratio of the two is 92:8, dissolve the sucrose in distilled water, heat, stir and mix until evaporated to dryness. Then, under the protection of a mixed gas of argon and 5% hydrogen by volume, it was treated at 650° C. for 2 hours to obtain carbon-coated lithium iron phosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com