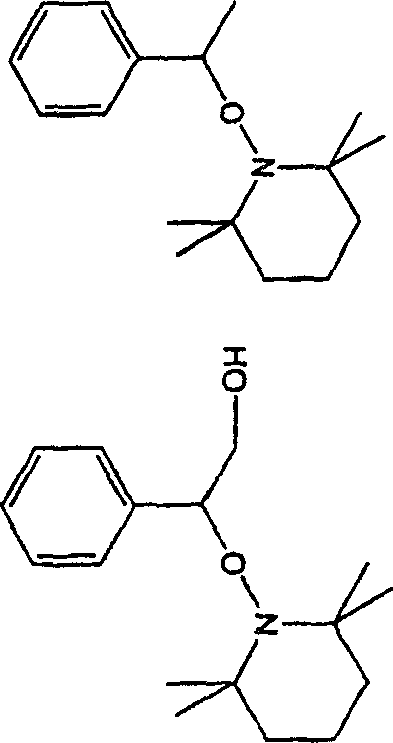
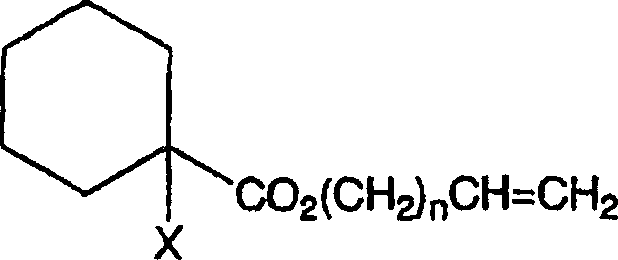
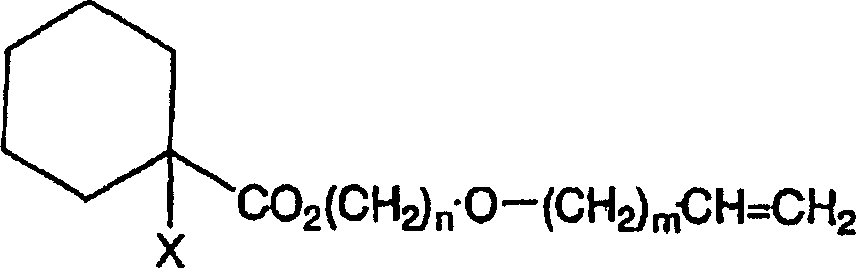Curable composition
A composition and polymer technology, applied in the field of vinyl polymers, can solve the problems of high viscosity, increased silanol-containing compounds, a large number of unsaturated silicone monomers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0616] A 10-liter separable flask equipped with a reflux condenser and stirrer was charged with CuBr (28.0 g, 0.195 mol). After purging with nitrogen, acetonitrile (559 ml) was added, and the mixture was stirred on an oil bath at 70°C for 15 minutes. Then, add butyl acrylate (1.00kg), 2,5-dibromodiethyl adipate (117g, 0.325mol) and pentamethyldiethylenetriamine [hereinafter referred to as triamine] (1.70ml, 1.41g, 8.14mmol), start the reaction. With continuous stirring and heating at 70° C., butyl acrylate (4.00 kg) was continuously added dropwise over 175 minutes. Additional triamine (8.50ml, 7.06g, 40.7mol) was added during the dropwise addition of butyl acrylate. 370 minutes after the reaction started, 1,7-octadiene (1.57L, 1.17kg, 7.10mol) and triamine (20.4ml, 16.9g, 97.7mmol) were added, and the whole mixture was stirred at 70°C for 220 minutes.
[0617] The reaction mixture was diluted with hexane, passed through an activated alumina column, and the volatile substanc...
preparation example 2
[0622]A 2-liter separable flask equipped with a reflux condenser and stirrer was charged with CuBr (22.4 g, 0.156 mol) and purged with nitrogen. Acetonitrile (112ml) was added, and the mixture was stirred on a 70°C oil bath for 30 minutes. Then, butyl acrylate (0.20kg), methyl 2-bromopropionate (86.9g, 0.520mol) and pentamethyldiethylenetriamine (hereinafter referred to as triamine) (0.19ml, 0.18g, 1.04 mmol), start the reaction. Under continuous stirring and heating at 70° C., butyl acrylate (0.80 kg) was continuously added dropwise over 150 minutes. Additional triamine (1.81 ml, 1.71 g, 9.88 mol) was added during the dropwise addition of butyl acrylate. The whole mixture was stirred with heating at 70°C for 230 minutes.
[0623] The reaction mixture was diluted with toluene, passed through an activated alumina column, and the volatile substances were evaporated under reduced pressure to obtain an alkenyl-terminated polymer (polymer [5]). The polymer [5] had a number aver...
Embodiment 1
[0626] 100 parts of polymer [4] obtained in Preparation Example 1, 3 parts of pentaerythritol triacrylate [(CH 2 =CHCOOCH 2 ) 3 CCH 2 OH], 50 parts of polymer [6] as a plasticizer and 100 parts of Calfine100 (Maruo Calcium product) as a filler were stirred and mixed. Then add 2 parts of γ-glycidoxypropyltrimethoxysilane and 1 part of Sn(IV) catalyst (dibutyltin diacetylacetonate), stir, degas the whole mixture, and mold to obtain about 2mm A thick sheet-shaped cured product and a plano-convex cured product with a maximum thickness of about 5 mm on a glass sheet. Curing was carried out by placing each product in a sunny indoor environment (near a window) for 2 days, and then placing it at 50°C for 3 days. After curing, the sheets were tested for residual tack (surface tack) by finger touch and then placed outdoors. Immediately after curing, pass through the glass with a xenon weather-o-meter (xenon weather-o-meter, Suga Testing Instruments product, SX120 type, illuminance ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com