EHEC 0157 shiga-like toxin IIA resisting subunit monoclonal antibody 5F3 light and heavy chain variable region gene and its application
A monoclonal antibody, Shiga-like toxin technology, applied in the direction of antibody, application, gene therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of anti-enterohemorrhagic Escherichia coli (EHEC) O157 Shiga-like toxin II toxin subunit Stx2A monoclonal antibody
[0025] 1. Immunization of Balb / c mice
[0026] Purified target protein Stx2A was used to immunize Balb / c mice (rats aged 6-8 weeks), 100ug / mouse, for the first immunization, 100μL of antigen was mixed with the same amount of complete Freund's adjuvant, and injected into the abdomen and groin of mice for subcutaneous immunization. After 14 days, the rats were immunized again, the dose and route of immunization were the same as the first immunization, and incomplete Freund's adjuvant was used. On the 28th day, additional immunization was given, 150ug / monkey, intraperitoneal injection.
[0027] 2. Preparation of Hybridoma Cells
[0028] 1) Collection of B lymphocytes: 3 days after the booster immunization, the eyes of the mice were extirpated and bled, and the serum was kept as a positive control. Take out the spleen by aseptic opera...
Embodiment 2
[0035] Embodiment 2 Cloning of anti-Stx2A monoclonal antibody 5F3 light and heavy chain variable region genes
[0036] 1. The cell line used is a high-affinity, high-specificity anti-Stx2A monoclonal antibody 5F3 hybridoma cell line obtained by the inventor using the above-mentioned method, and the antibody secreted by it does not interact with various Escherichia coli enterotoxins such as CT and LT. Antigen binding, the subtype of antibody molecules secreted is IgG1k.
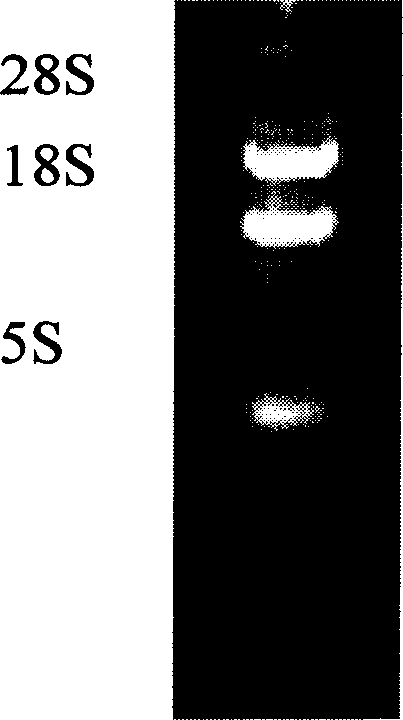
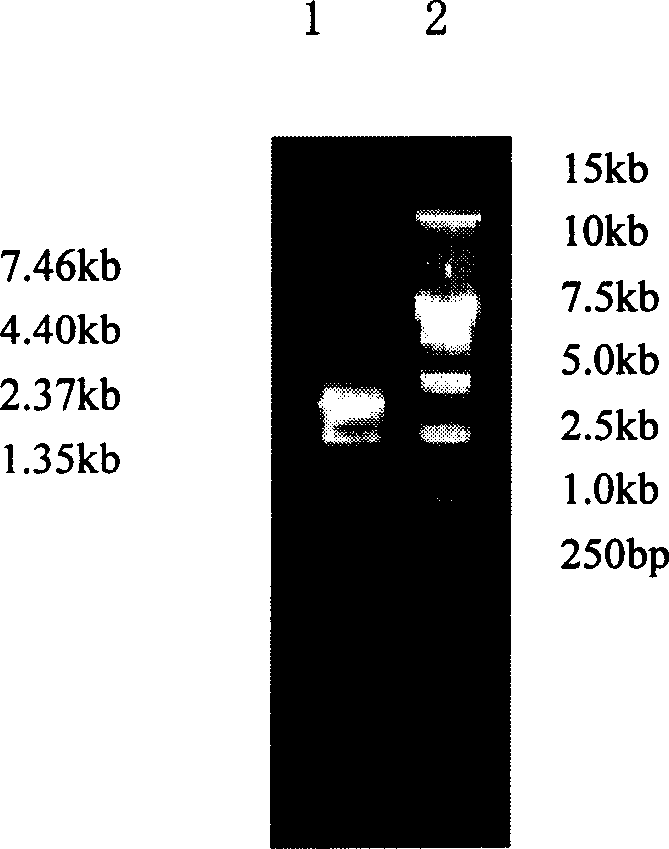
[0037] 2. Take the 5F3 hybridoma cells in logarithmic growth phase (2×10 6 ), using guanidine isothiocyanate one-step method (TaKaRa Company) to extract total RNA, and take a small amount for quantification by UV spectrophotometer and detection by 1% agarose gel electrophoresis. Subsequently, oligo(dT)15 (TaKaRa Company) was used as a random primer to generate cDNA by reverse transcription. Then, the light and heavy chain variable region genes of monoclonal antibody 5F3 were amplified specifically using gene...
Embodiment 3
[0099] Example 3 Construction and expression of the ScFv form antibody of the anti-Stx2A monoclonal antibody (using the ScFv expression kit of Pharmacia Company)
[0100] Put VH, VL and Linker(G 4 S) 3 After the primers are equimolarly mixed, the polymerization and filling reactions are carried out, so that VH and VL are linked together by LinkerDNA to form ScFvDNA. Then, restriction enzyme cutting site primers were added to amplify the polymerized ScFv and introduce a Sfi I restriction enzyme cutting site at the 5' end and a Not I restriction enzyme cutting site at the 3' end. Cloned into the modified secretory expression vector pCANTAB6His, transformed into Escherichia coli HB2151 for inducible expression. Identification by restriction enzyme digestion and DNA sequencing analysis confirmed that the gene was constructed correctly. After the expressed protein was purified by affinity chromatography, the expressed product was analyzed and detected by SDS-PAGE electrophoresis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com