Anti-DON single-chain antibody ScFv and preparing method and application thereof
A single-chain antibody and anti-vomiting technology, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of antibody hybridoma cell lines that are prone to mutation, expensive, and cell line instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Establishment of anti-vomitin monoclonal antibody McGill078 hybridoma cell line and preparation of anti-vomitoxin monoclonal antibody
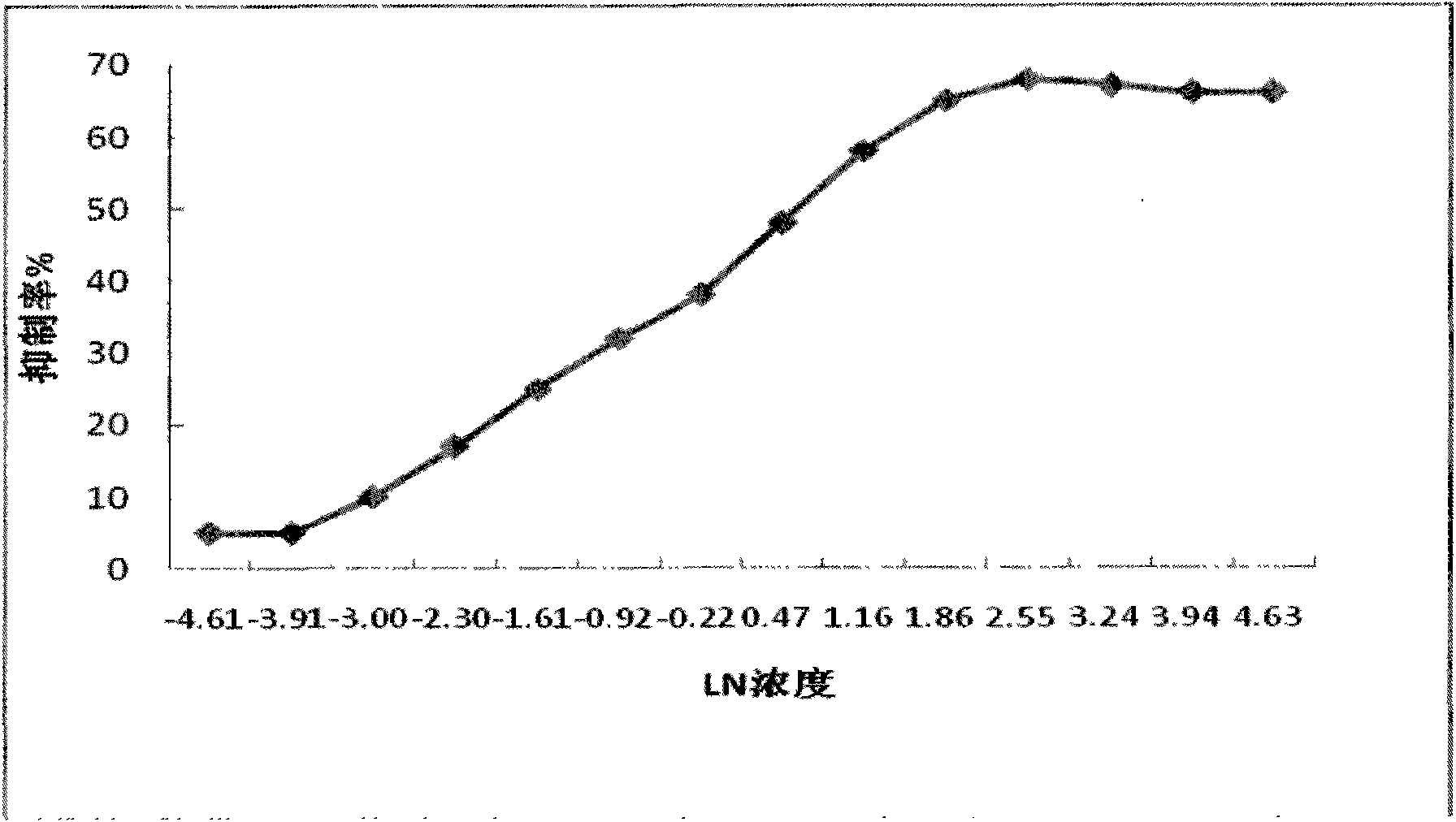
[0026] The antigen was synthesized by literature method (Casale WL, Journal of Agriculture and Food Chemistry, 1988, 36:663-668), and the hybridoma cell line was established according to conventional methods (Wu Jianxiang, Acta Microbiologica Sinica, 2000, 40 (6 ): 638-645) named McGill078, the McGill078 hybridoma cell line was cultured, subcultured, resuspended and inoculated in the abdominal cavity of mice, and about 15 ml of ascites was collected after the abdomen was swollen 10 days later. The anti-vomitin antibody in rat ascites was purified by rapid batch adsorption method, and concentrated by ultrafiltration membrane dialysis; the concentration of this monoclonal antibody was determined to be 9.2 mg / ml, and the antibody type was IgG1 (γ 1 , κ), with good antigen specificity and antigen affinity.
Embodiment 2
[0027] Example 2. Cloning of heavy chain and light chain variable region genes of anti-vomitin monoclonal antibody McGill078
[0028] 1. The monoclonal hybridoma cell line McGill078 used can secrete antibodies that specifically recognize vomitoxin DON, and the molecular subtype of the secreted antibodies is IgG 1 , whose heavy and light chain isotypes are γ 1 / K.
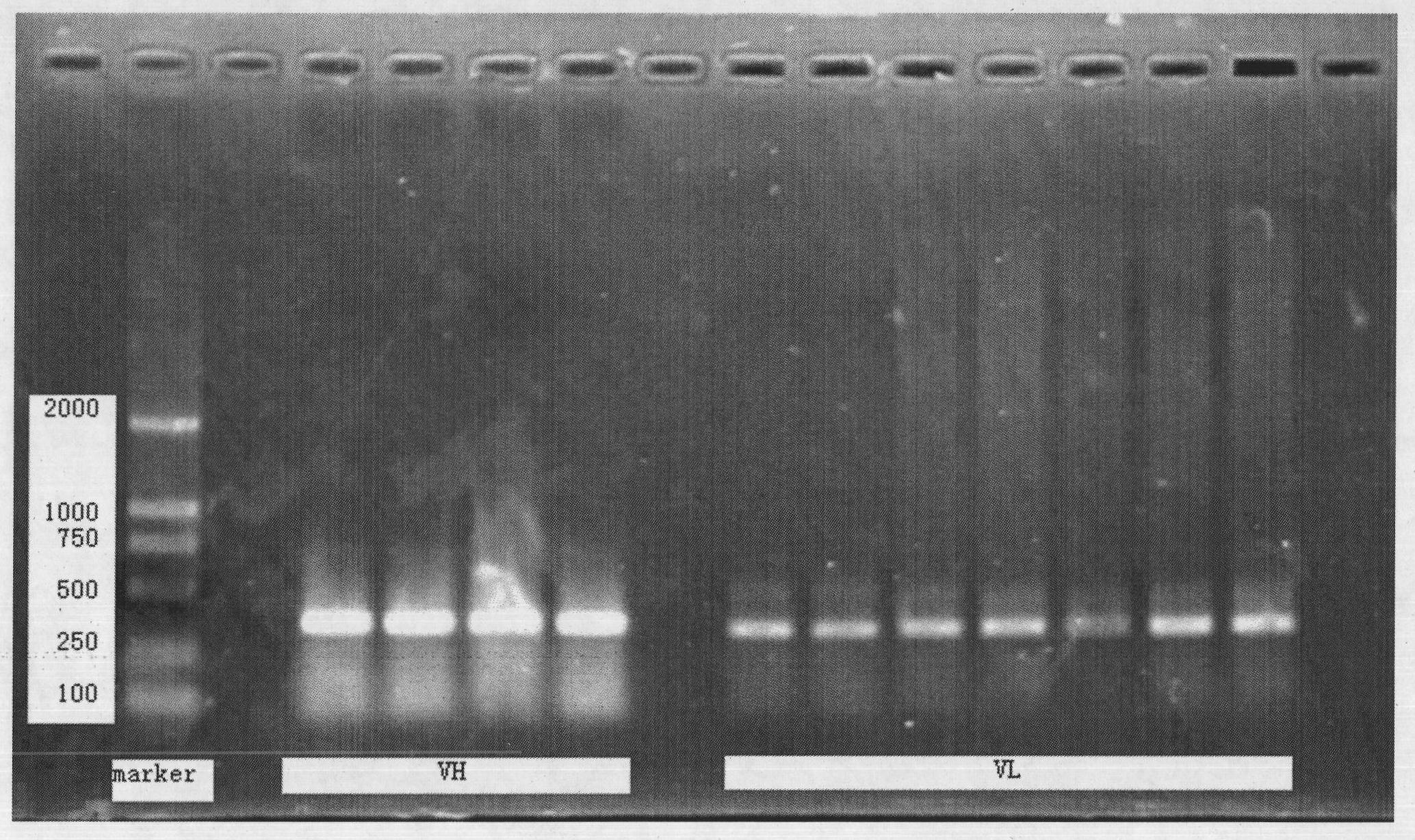
[0029] 2. Take the McGill078 hybridoma cells (2×10) in the logarithmic growth phase 6), using Trizol (Invitrogen) reagent to extract total RNA, isolate and purify mRNA (PolyATtract mRNA Isolation Systems, Promega), according to Invitrogen kit (SuperScript III First-Strand Synthesis System for RT-PCR), with Oligo(dT)20 as primer , RT-PCR reverse transcription to synthesize cDNA, then amplify the light and heavy chain variable region genes with specific primers, 1% agarose gel electrophoresis, recover the fragments with gel recovery test kit (promega company), TA clone insert pMD18- T vector, sequence determination...
Embodiment 3
[0098] Example 3. Construction and expression of single-chain antibody ScFv
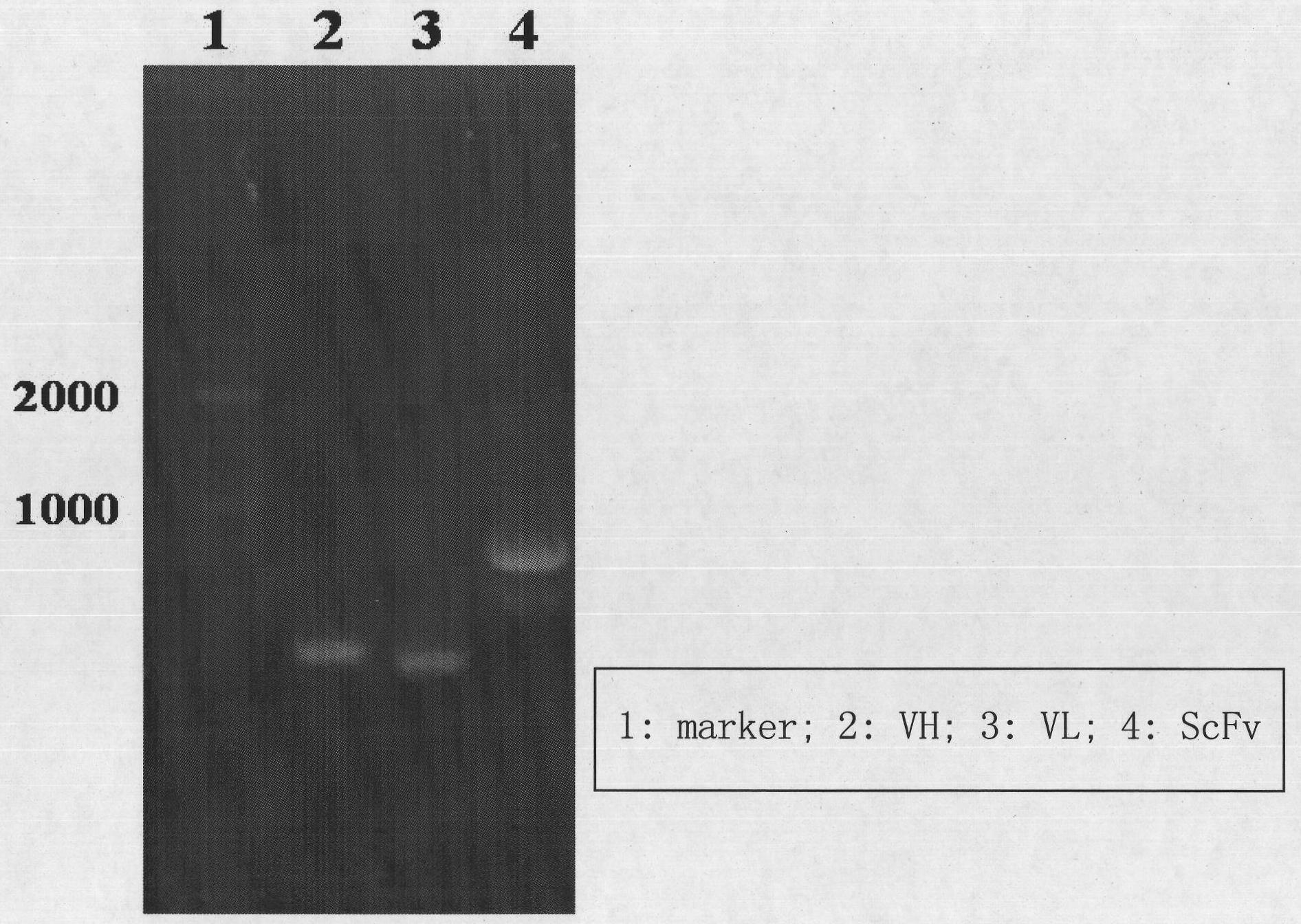
[0099] ScFv was assembled by overlapping extension (SOE) with a DNA fragment encoding a hydrophilic polypeptide linker (G 4 S) 3 The cloned antibody light and heavy chain variable region genes are connected to form a 5' VH-Lingker-VL3' format. ScFv and expression vector pET26b (+) (Novagen Company) were digested with restriction endonucleases BamHI and HindIII, connected with T4 ligase, transformed into Escherichia coli TOP10F' for expression, and then targeted for expression in the form of inclusion bodies (IBS) ScFv expression system, optimization of IPTG-induced expression conditions, inclusion body isolation, denaturation, and renaturation. The results showed that ScFv was successfully expressed in Escherichia coli.
[0100] The relevant operation steps are as follows:
[0101] (1) Cloning of VH and VL genes in pMD18-T plasmid
[0102] pMD 18T-VH template (50ng)
[0103] (Or pMD18T-VL) (50n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com