Method for preparing z-deoxy-D-glucose
A technology of glucose and dideoxy, applied in the direction of deoxy/unsaturated sugar, etc., can solve the problems of low selectivity and low yield, and achieve the effects of good selectivity, good production safety and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
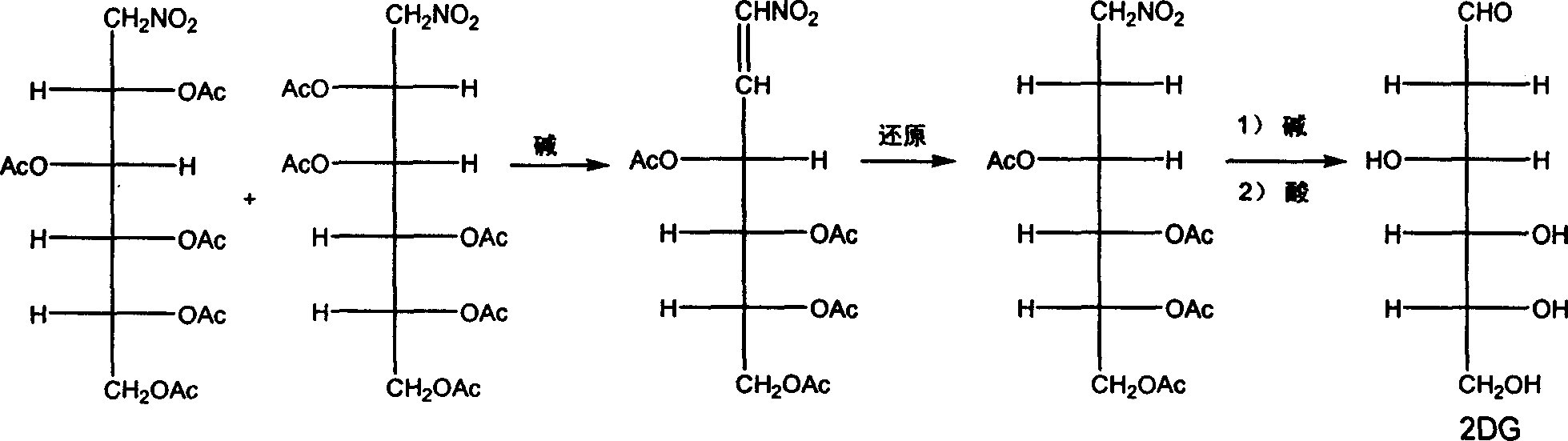
[0028] 1), the preparation of D-arabo-tetraacetyl-1-nitrohexene-1:
[0029] In 50 ml of n-hexane, add 3.53 grams of 1-nitro-1-deoxy-D-mannose pentaacetate, 3.8 grams of sodium bicarbonate, and react at 60°C; after the reaction is completed, cool, filter, and spin the filtrate under reduced pressure The solvent was removed by evaporation, and the residue was recrystallized from 20-40 ml of ethyl acetate to obtain 2.69 g of a white flaky solid, namely D-arabo-tetraacetyl-1-nitrohexene-1, with a yield of 89.1%.
[0030] 2), the preparation of 1-nitro-1,2-dideoxy-D-arabo-hexose tetraacetate:
[0031] Add 1.85 g of D-arabo-tetraacetyl-1-nitrohexene-1 to 25 ml of solvent (methanol:ether volume ratio 1:1), add 0.15 g of lithium borohydride and 0.50 g of aluminum oxide as an additive; Stir the reaction at 30°C until the raw material disappears, filter, and neutralize the filtrate to PH6-8 with 5% hydrochloric acid by weight, remove the solvent by rotary evaporation under reduced pres...
Embodiment 2
[0036] 1), the preparation of D-arabo-tetraacetyl-1-nitrohexene-1:
[0037] In 50 ml of cyclohexane, add 3.53 grams of 1-nitro-1-deoxy-D-mannose pentaacetate, 4.0 grams of sodium carbonate, and react at 50 ° C; after the reaction is completed, cool, filter, and spin the filtrate under reduced pressure The solvent was removed by evaporation, and the residue was recrystallized through 20-40 ml of ether to obtain 2.52 g of a white flaky solid, ie, D-arabo-tetraacetyl-1-nitrohexene-1, with a yield of 83.4%.
[0038] 2), the preparation of 1-nitro-1,2-dideoxy-D-arabo-hexose tetraacetate:
[0039] Add 1.85 g of D-arabo-tetraacetyl-1-nitrohexene-1 to 30 ml of solvent (methanol:chloroform volume ratio 1:1), add 0.20 g of potassium borohydride, and stir the reaction at 50°C until the raw material disappears , with a weight concentration of 5% hydrochloric acid to neutralize to PH6-8, remove the solvent by rotary evaporation under reduced pressure, add 30 ml of water, extract with chlo...
Embodiment 3
[0044] 1), the preparation of D-arabo-tetraacetyl-1-nitrohexene-1:
[0045] In 50 ml of toluene, add 3.53 grams of 1-nitro-1-deoxy-D-mannose pentaacetate, 4.8 grams of potassium carbonate, and react at 80°C; after the reaction is completed, cool, filter, and remove the filtrate by rotary evaporation under reduced pressure solvent, the residue was recrystallized from 20-40 ml of ethyl acetate-petroleum ether (10 ml of ethyl acetate), and 2.67 g of white flaky solids were obtained, that is, D-arabo-tetraacetyl-1-nitrohexene- 1. The yield is 88.4%.
[0046] 2), the preparation of 1-nitro-1,2-dideoxy-D-arabo-hexose tetraacetate:
[0047] Add 1.85 g of D-arabo-tetraacetyl-1-nitrohexene-1 to 30 ml of solvent (methanol: tetrahydrofuran volume ratio 1:5), add 0.12 g of sodium borohydride, and stir the reaction under reflux until the raw materials disappear. Neutralize to PH6-8 with 5% hydrochloric acid by weight, remove the solvent by rotary evaporation under reduced pressure, add 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com