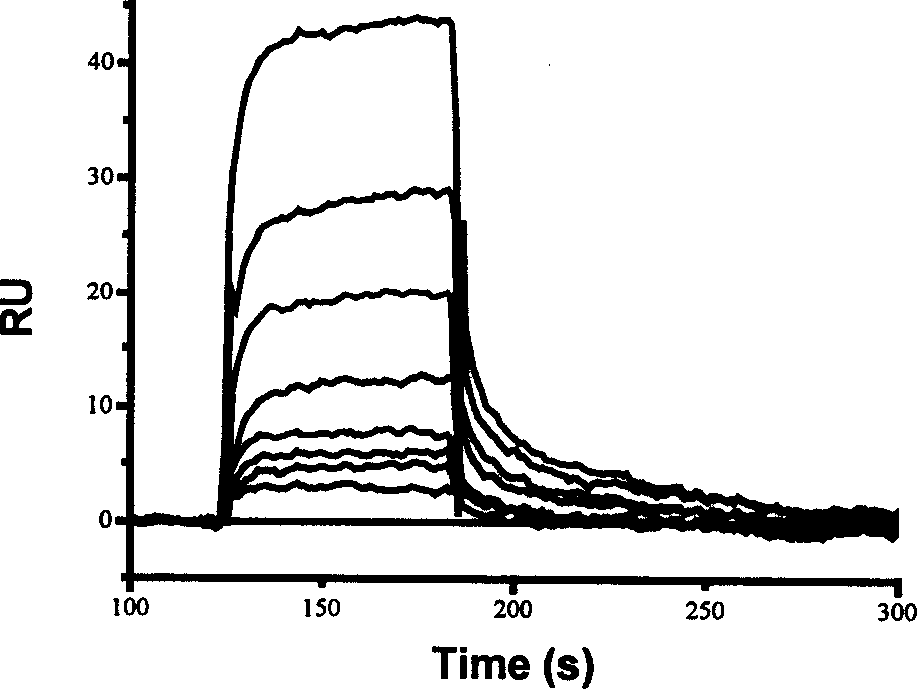
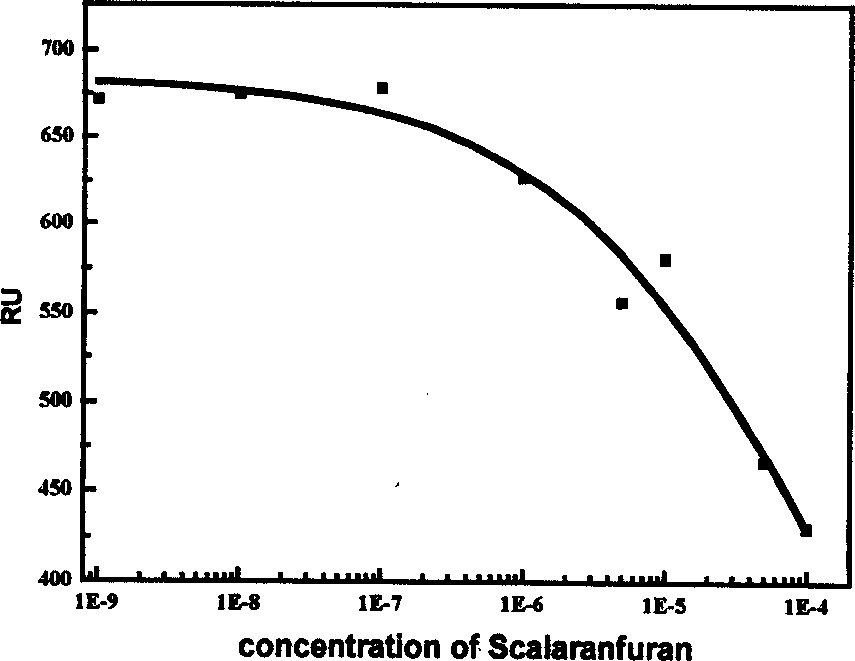
Use of compound scalarafuran
A compound and application technology, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as the uncertainty of whether the compound has a direct effect, the toxic and side effects of HIV drug-resistant reverse transcriptase and protease inhibitors, and the lack of drugs for IN inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Frozen fresh sponge (dry weight 138 g) was chopped, ultrasonically extracted with analytical grade acetone until colorless, the acetone extract was concentrated under reduced pressure (temperature below 30°C), the concentrate was suspended in 500 ml of distilled water, extracted with ether, The ether extract was concentrated under reduced pressure to obtain 5.4 grams of extract, which was subjected to silica gel (200-300 mesh) column chromatography [petroleum ether (60-90°C) / ethyl acetate gradient elution], and the eluents were combined Seven fractions were obtained, and the fifth fraction eluted by petroleum ether (60-90° C.) / ethyl acetate 78 / 12 was subjected to gel LH-20 (chloroform elution) column chromatography, and then passed through a silica gel column Chromatography, eluting with petroleum ether (60-90°C) / diethyl ether 75 / 25, isolated a pure compound as colorless crystals (8.2 mg). 1 H-NMR and 13 The C-NMR data are as follows: 1 H NMR (CDCl 3 )δ: 7.53(s, 1H, ...
Embodiment 2
[0078] Example 2 Construction of fusion protein plasmid pGEX-4T-1-IN (F185K) of GST-tagged HIV-1 integrase (IN, integrase):
[0079] (1) Experimental method:
[0080] 1) Obtain the DNA fragment of IN from the pUC18-IN plasmid by PCR technology (the gene sequence is based on AF040373 (GENEBANK)).
[0081] Primer: FW: 5′-ata tgg atc ctt ttta gat gga ata gat-3′
[0082] RV: 5′-ata tct cga gct aat cct cat cct g-3′
[0083] Denaturation at 94°C for 5min, then cycle: 94°C for 45s, 55°C for 45s, 72°C for 1min30s, repeat 29 cycles, then 72°C for 10min.
[0084] 1% agarose gel electrophoresis was used to identify PCR products and recover PCR fragments.
[0085] 2) Clone the IN PCR fragment into the vector pGEX-4T-1: the IN PCR product and pGEX-4T-1 were digested with BamHI and XhoI respectively; the digested product was recovered with a gel recovery kit; Ligation reaction; the ligation product was transformed into DH5α competent cells and spread on a plate containing ampicill...
Embodiment 3
[0093] Example 3 Expression and purification of GST-tagged HIV-1 integrase (GST-IN):
[0094] (1) Experimental method
[0095] 1) Transfer the plasmid pGEX-4T-1-IN(F185K) into the expression strain BL21(DE3), select the correct clone and culture it in 10ml ampicillin LB medium (yeast extract 5g / L, tryptone 10g / L and NaCl10g / L, the concentration of ampicillin is 100mg / L), transferred to fresh ampicillin LB medium at a ratio of 1:100, and cultured with shaking until OD 600 When the value is between 0.6-0.8, add IPTG to a final concentration of 0.2mM and lower the culture temperature to 25°C to induce protein expression for 5-7 hours. Bacteria were collected by centrifugation at 5,000 rpm for 10 min, suspended and washed with PBS buffer, and then centrifuged again, and the collected bacteria were stored in a -70°C refrigerator until use.
[0096] 2) Suspend the bacteria to be used in 20ml PBS, sonicate the bacteria, centrifuge at 15,000rpm for 30min, take the supernatant and Gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com