Gene engineered poly-valence subunit vaccine of Hp AhpC and preparation method thereof
A genetically engineered vaccine, Helicobacter pylori technology, applied in chemical instruments and methods, bacterial antigen components, drug combinations, etc., can solve problems such as difficult large-scale cultivation, unfavorable vaccine industrialization preparation, and harsh conditions for Hp bacterial culture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1 Cloning of Helicobacter pylori AhpC, NapA coding genes and extramolecular adjuvant LTB, CTB genes
[0117] 1. Helicobacter pylori is derived from clinical isolates from patients in the Gastroenterology Department of Southwest Hospital (strain number: CQ9803)
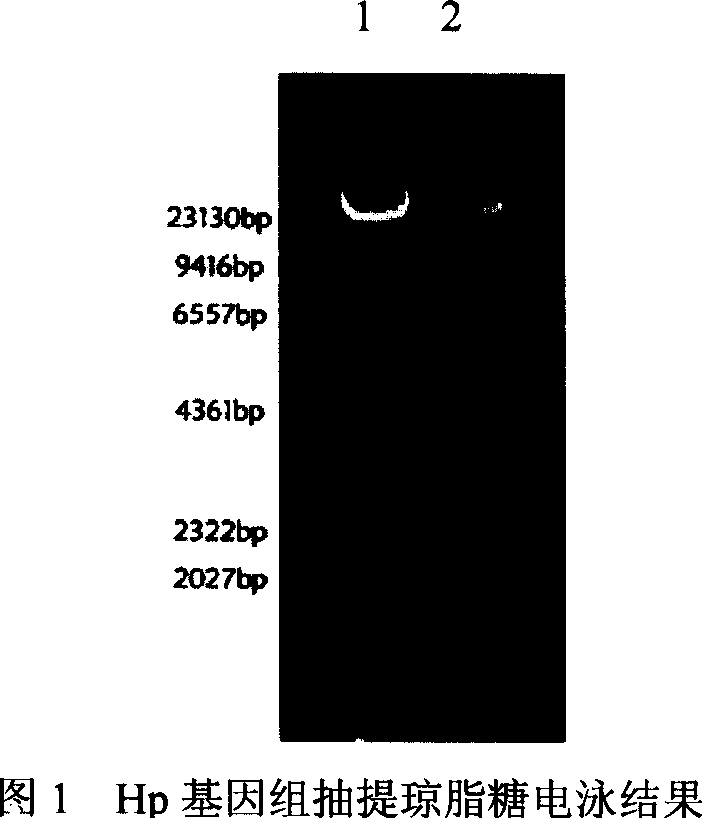
[0118] 2. Take out the preserved Hp strain (CQ 9803) from the liquid nitrogen tank and spread it on the special solid medium for Hp, at 37°C, containing 5% O 2 , 85%N 2 , 10% CO 2 Conditioned for 72h. The genome extraction kit was used to extract the Hp genome (shown in Figure 1).
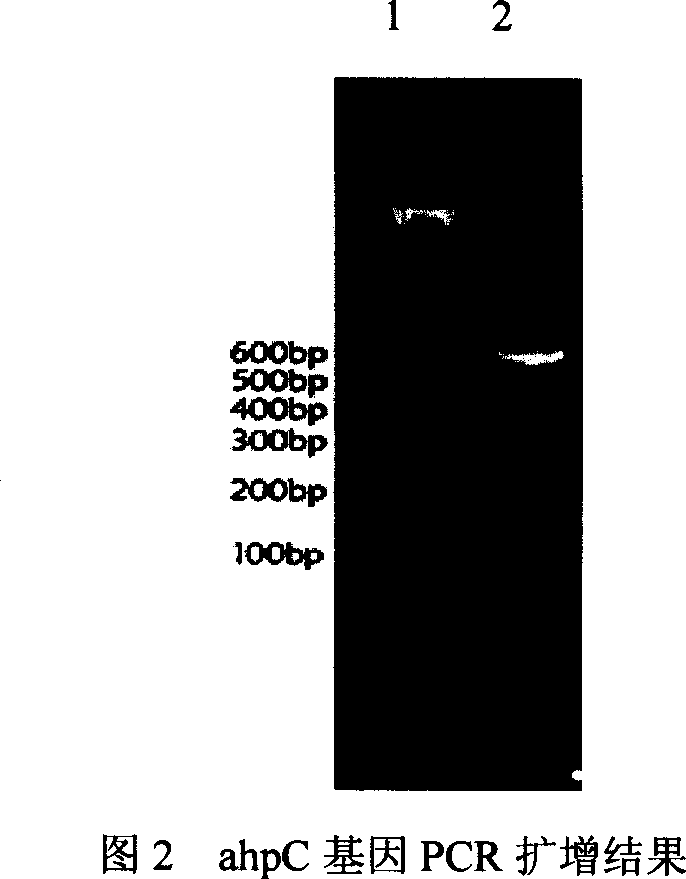
[0119] 3. The coding genes of AhpC and NapA were respectively amplified from the Hp genome by PCR method.
[0120] 1) The primers were designed and synthesized as follows (the restriction site and linker base sequence are underlined)
[0121] According to the gene sequence published by GenBank and the principles of primer design, the corresponding primers were designed and restriction sites were introduced. The followin...
Embodiment 2
[0210] Example 2 Acquisition of Intramolecular Adjuvant Fusion Gene AhpC-LTB(AL)
[0211] Primer design and synthesis
[0212] AhpC P1 5'-CG CATATG TTAGTTACAAAAACTTGCC-3'
[0213] Nde I
[0214] P2' 5'- GCTACCTCCGCCTCC AAGCTTAATGGAAT
[0215] Linker
[0216] LTB P5” 5’- GGAGGCGGAGGTAGC GCTCCCCAGTCTATT-3'
[0217] linker
[0218] P6 5'- CTCGAG GTTTTCCATGCTGATTGC-3'
[0219] wxya
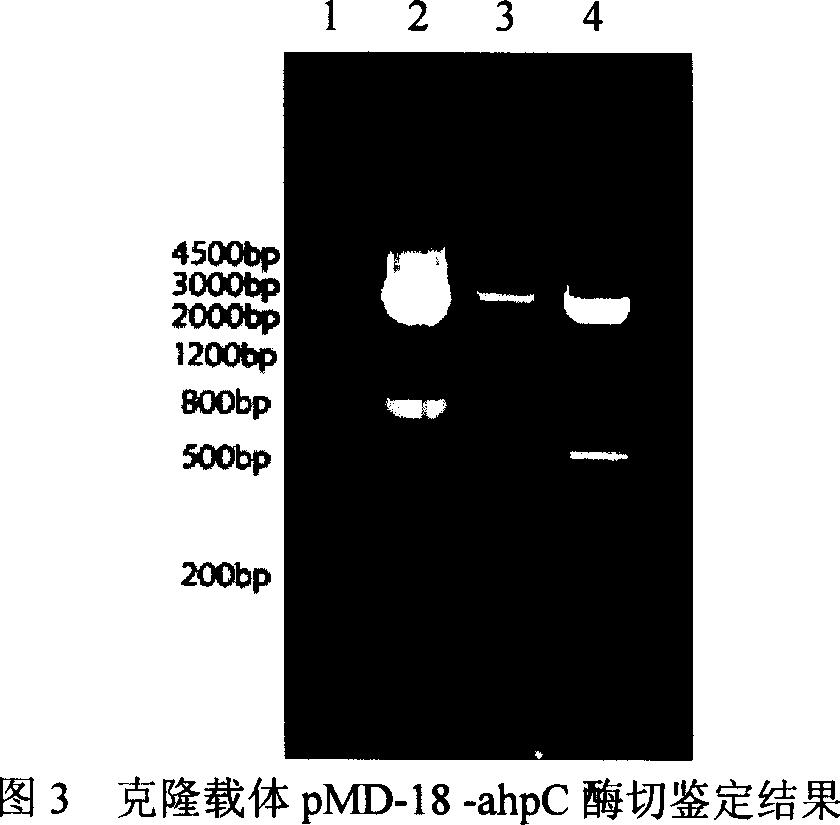
[0220] Using the AhpC and LTB recovered in Example 1 as templates, use P1 and P2', P5" and P6 primers to amplify AhpC and LTB genes respectively, and the bacterial genome extraction is carried out on a daily basis with a spin-column type bacterial genomic DNA extraction kit The PCR amplification system is: template DNA 1μl; 10×PCR buffer 5μl; dNTPs (10mmol / L) 4μl; primers (0.025mmol / L) 1μl each; Taq DNA polymerase (5u / μl) 0.5μl; water to a final volume of 50 μl.
[0221] T...
Embodiment 3
[0225] Example 3 Acquisition of Intramolecular Adjuvant Fusion Gene AhpC-CTB(AC)
[0226] Primer design and synthesis
[0227] AhpC P1 5'-CG CATATG TTAGTTACAAAAACTTGCC-3'
[0228] Nde I
[0229] P2' 5'- GCTACCTCCGCCTCC AAGCTTAATGGAAT
[0230] Linker
[0231] CTB P7” 5’- GGAGGCGGAGGTAGC ATTAAATTAAAATTTGGT-3'
[0232] linker
[0233] P8 5'- CTCGAG ATTTGCCATACTAATTGCG-3'
[0234] wxya
[0235] Using the AhpC and CTB recovered in Example 1 as templates, using P1 and P2', P7" and P8 as primers, respectively amplify the AhpC and CTB genes, and extract the bacterial genome by days. The PCR amplification system is: template DNA 1μl; 10×PCR buffer (containing magnesium chloride) 5μl; dNTPs (10mmol / L) 4μl; primers (0.025mmol / L) each 1μl; Taq DNA polymerase (5u / μl ) 0.5 μl, add deionized water to a final volume of 50 μl.
[0236] The reaction system was mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com