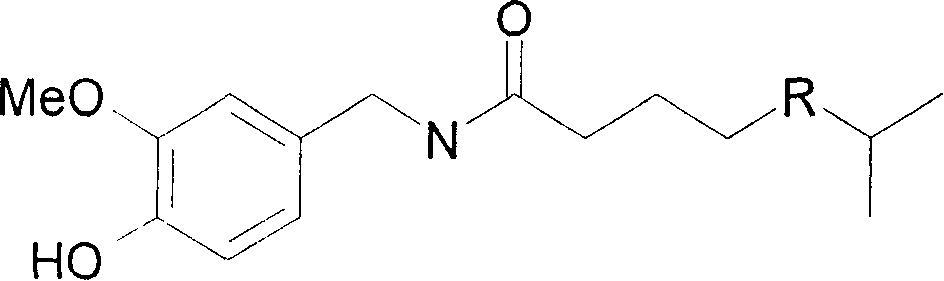
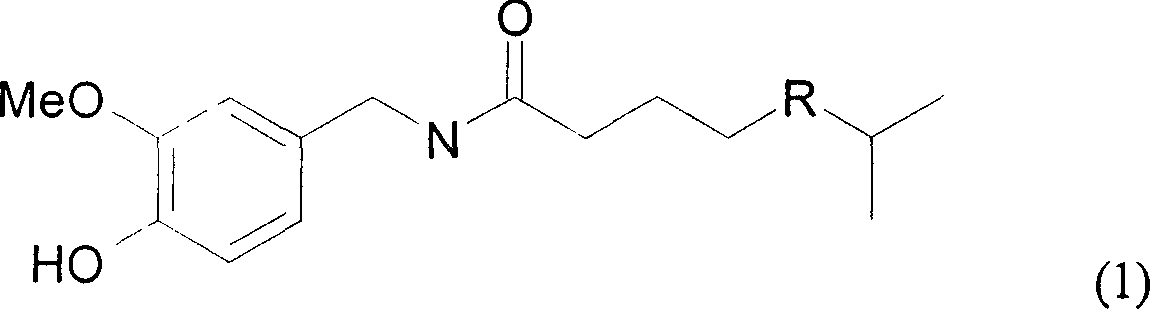
Capsicine chemical synthesis and purification method
A technology of capsaicin and compounds, which is applied in the field of chemical synthesis and purification of capsaicin and its analogues, can solve the problems of harsh synthetic conditions of capsaicin and unsuitability for industrial production, and achieve easy industrial production, low cost, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
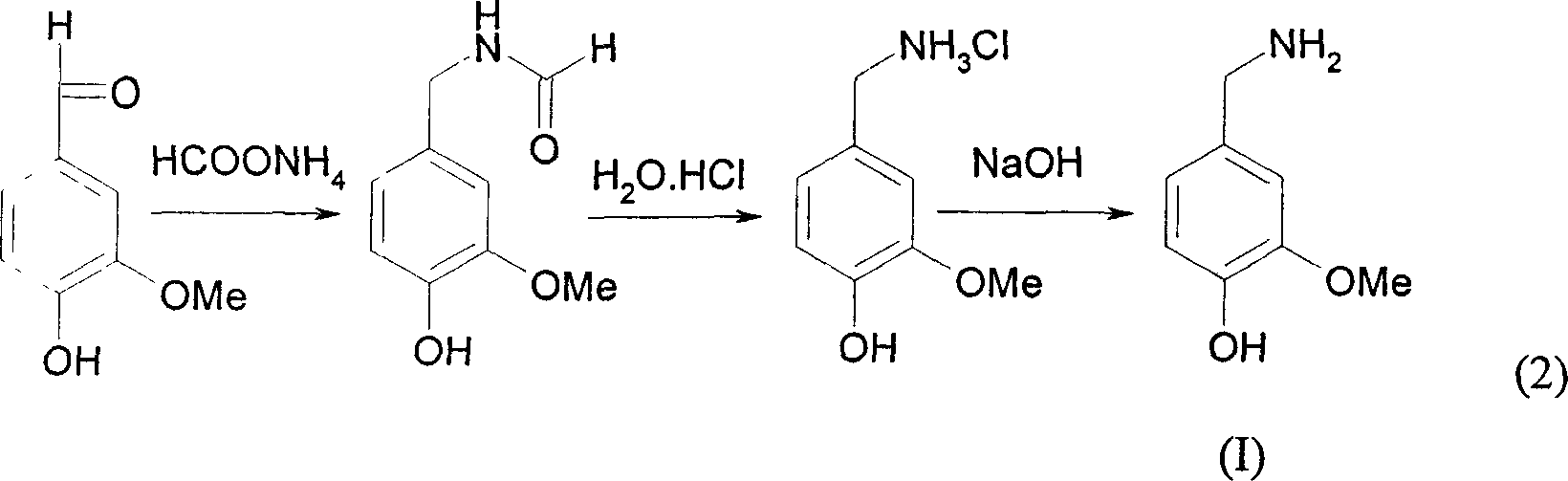
[0034] Embodiment 1: the synthesis of vanillylamine:
[0035] In a 250ml three-neck flask equipped with a reflux condenser and a stirring device, add vanillin (7.6g, 50mmol) and ammonium formate (10g, 160mmol), heat to 150°C for 5h, and after cooling, add concentrated Hydrochloric acid (6ml), reflux for 1 hour, add 70ml of ethanol to crystallize the hydrochloride of vanillylamine to obtain the hydrochloride of vanillylamine, recrystallize once with 95% ethanol to obtain pure hydrochloride of vanillylamine, m.p.216-218℃ , the yield was 49.8%. Dissolve 1.8g of vanillylamine hydrochloride in 30ml of water, add 2M NaOH (10.0ml) dropwise under vigorous stirring, a white precipitate appears in the solution, after suction filtration, water washing and drying, white vanillylamine is obtained, m.p.134-136°C, yield The rate is 56%.
[0036] EI-MS: 153 (M +, 100), 136(70), 122(54), 110(23), 93(20), 65(16), 30(19)
Embodiment 2
[0037] Embodiment 2: the synthesis of vanillylamine:
[0038] In a 250ml three-neck flask equipped with a reflux condenser and a stirring device, add vanillin (15.2g, 0.1mol) and ammonium formate (20g, 0.32mol), heat to 150°C for 5 hours, and after cooling, add Add concentrated hydrochloric acid (12ml), reflux for 1h, then add 70ml of ethanol to crystallize the hydrochloride of vanillylamine, and recrystallize the obtained light brown crystals with 95% ethanol once to obtain pure vanillylamine hydrochloride, which is light Pink needle crystal, m.p.216-218°C, yield 50%. Dissolve 3.5g of vanillyl amine hydrochloride in 50ml of water, add dropwise 2M NaOH (16.4ml) under vigorous stirring, a white precipitate appears in the solution, after suction filtration, water washing and drying, white vanillyl amine is obtained, m.p.134-136°C, harvested The rate is 44.5%. The characterization data are the same as in Example 1.
Embodiment 3
[0039] Embodiment 3: (6-carboxyhexyl) triphenylphosphine bromide is synthesized
[0040] In a 250ml three-necked flask equipped with a stirring and reflux condenser, add 6-bromo-hexanoic acid (5.16g, 24mmol), triphenylphosphine (6.94g, 24mmol), toluene (20ml), heat to reflux for 10h, and after cooling, After filtration, the resulting precipitate was recrystallized with chloroform to obtain white crystals, m.p. 201-203°C, yield 94.2%.
[0041] 1 H NMR (600MHz): δ1.47-1.55 (6H, m, C 2,4,6 -H); 2.16(2H,t,C 2 -H); 3.58(2H, m, C 6 -H); 7.75-7.92 (15H, m, Ar-H); 12.02 (1H, brs, COOH).
[0042] ESI-MS: pos(377); neg(535, 537, 455, 457)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com