Synthesis method of imide substituted bridge ring compound
A synthetic method and imide-based technology, applied in the direction of organic chemistry, can solve the problems of low yield and long reaction time, and achieve the effects of lower reaction temperature, simple operation, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Diels-Alder reaction of 1-butyrolactam-3-phenylpropenone and cyclopentadiene
[0024] Add 5 mL of dichloromethane and 0.22 g (1 mmol) of 1-butyrolactam-3-phenylpropenone into a 50 mL one-necked flask, then add 0.15 g (0.5 mmol) of zinc iodide and 0.5 mL (5 mmol) of cyclopentanediene ene, placed in an ultrasonic water tank, subjected to ultrasonic radiation (40KHz, 50W) for 30min, added 20mL of saturated sodium bicarbonate solution, extracted with dichloromethane (3×5mL), washed twice with water, dried, concentrated, and subjected to column chromatography (petroleum Ether: ethyl acetate = 9: 1 V / V) to obtain 0.2 g of white needle-like crystals with a melting point of 75-77° C. and a yield of 71%. IR: ν=2989, 1730, 1686, 1458, 1346, 1328, 1286, 1247, 1219, 1020, 793cm -1 . 1 H NMR (400MHz, CDCl 3 ): δ=1.55(m, 1H, H7), 1.96(m, 1H, H7'), 2.02(m, 2H, H17), 2.58(m, 2H, H16), 2.98(s, 1H, H1), 3.38(m, 2H, H16), 3.77(m, 2H, H18), 4.20(dd, J=3.4, 4.9Hz 1H, H5), 5.90...
Embodiment 2
[0025] Example 2 Diels-Alder reaction of 1-succinimide-2-butenone and cyclopentadiene
[0026] Add 5mL of 1-butyl-3-methylimidazolium tetrafluoroborate and 0.17 grams (1mmol) of 1-succinimidyl-2-butenone into a 50mL one-necked flask, then add 0.15 grams (0.5mmol ) zinc iodide and 0.5mL (5mmol) cyclopentadiene, placed in an ultrasonic water tank, subjected to ultrasonic radiation (40KHz, 50W) for 25min, extracted with toluene (3×5mL), washed twice with water, dried, concentrated, and passed through the column layer Analysis (petroleum ether: ethyl acetate = 2:1 V / V) gave 0.22 g of white needle-like crystals with a melting point of 88-89° C. and a yield of 93.3%. IR: ν=2975, 2872, 1795, 1746, 1709, 1429, 1371, 1317, 1253, 1183cm -1 . 1 H NMR (400MHz, CDCl 3 ): δ=1.18 (d, J=7.2, 3H, Me-6), 1.46 (dd, J=9, 1.6Hz, 1H, H-7 a ), 1.63 (d, J=8.8, 1H, H-7 b ), 2.05(m, 1H, H-1), 2.54(s, 1H, H-6), 2.8(s, 4H, H-11, H-12), 3.07(s, 1H, H-4), 3.24(dd, J=3.6, 4Hz, 1H, H-5), 5.91(dd, J=2.6...
Embodiment 3
[0027] Example 3 Diels-Alder reaction of 1-succinimide-2-butenone and cyclopentadiene
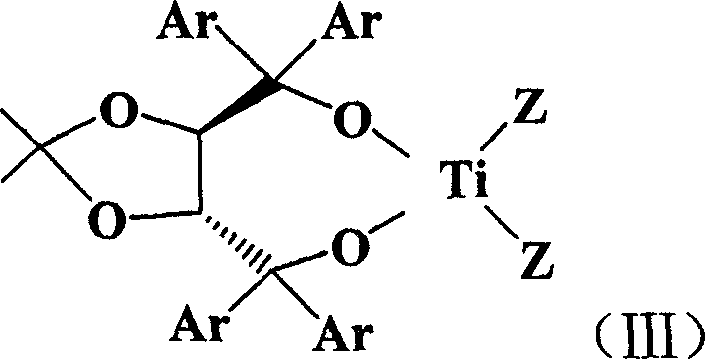
[0028] In a nitrogen stream, 0.4 g of dried activated 4A molecular sieve powder and optically active (2R, 3R)-(-)-1,1,4,4-tetrakis-(1-naphthyl)-2,3-( 0.15 g (0.22 mmol) of acetonide)-1,4-butanediol was placed in a 25 mL reactor and sealed. Fill the reaction system with nitrogen to make the reaction system anaerobic and absolutely dry, add 3mL dichloromethane with a syringe, mix and stir thoroughly, and then add 0.2mL TiCl 2 (OPr i ) 2 Add the toluene solution (0.87mol / L) into the reaction system, stir at room temperature for 1 hour, then lower the temperature to -10°C, and dissolve 0.17 g (1 mmol) of 1-succinimide-2-butenone in 4 mL Dichloromethane was first added to the reaction system, and after stirring for a few minutes, 1mL (10mmol) of cyclopentadiene was added, placed in an ultrasonic water tank, subjected to ultrasonic radiation (40KHz, 50W) for 30min, and 20mL of saturated sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com