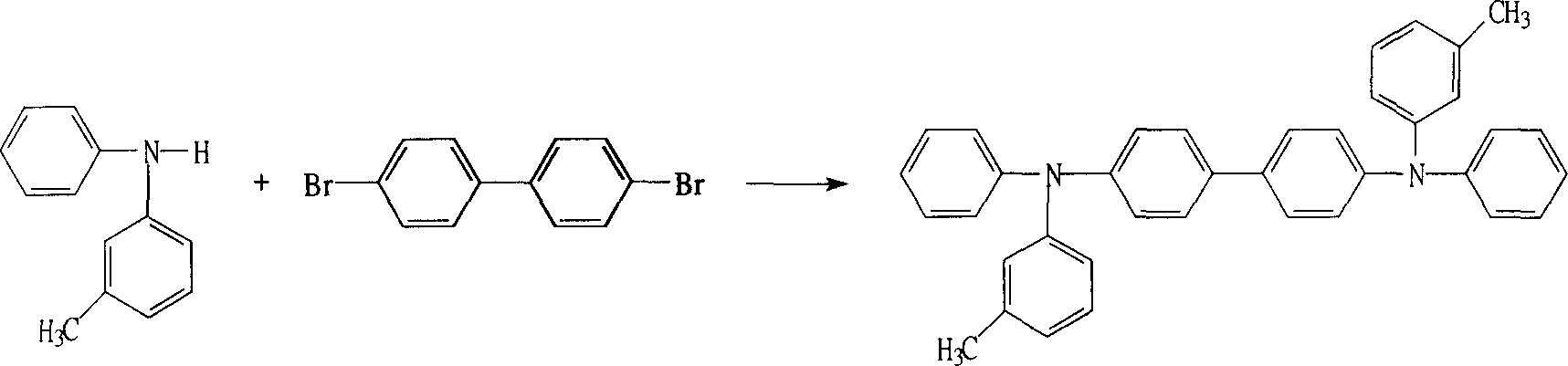
N,N'-bi(3-methylphenyl)-N,N'-diphenylbenzidine preparation method
A technology of diphenylbenzidine and methyldiphenylamine, applied in the field of N, can solve the problems of difficult preparation of special ligands, narrow active temperature range, difficult product separation, etc., achieve excellent hole transport performance, and reduce production costs , the effect of improving the reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 36.6 grams (0.20mol) of 3-methyldiphenylamine and 25.9 grams (0.083mol) of dibromobiphenyl in a 500mL three-necked flask, 200ml of toluene, in 0.366 grams of cuprous iodide (1.92mmol), N, N- Under the catalysis of 0.23 grams (1.92 mmol) of dimethylaniline (1.92 mmol) and 0.299 grams (1.92 mmol) of 4,4'-bipyridine, the reaction was carried out in a potassium hydroxide system for 5 hours to obtain the crude product through decolorization, washing, drying and distillation, and then 80 ml of toluene, hexa 80ml of alkane and 80ml of methanol were mixed for purification, and finally 39 grams of finished product (purity 99.8%) were obtained, and the reaction yield was 91%.
Embodiment 2
[0026] Add 36.6 grams (0.20mol) of 3-methyldiphenylamine and 25.9 grams (0.083mol) of dibromobiphenyl in a 500mL three-necked flask, 200ml of toluene, in 0.366 grams of cuprous iodide (1.92mmol), N, N- Under the catalysis of 0.23 grams (1.92mmol) of dimethylaniline, reacted in the potassium hydroxide system for 8 hours and obtained the crude product through decolorization, washing, drying and distillation, then purified by mixing 80ml of toluene, 80ml of hexane, and 80ml of methanol, and finally obtained 30 grams of finished product ( The purity is 99.0%), and the reaction yield is 70%.
Embodiment 3
[0028] Add 36.6 grams (0.20mol) of 3-methyldiphenylamine and 25.9 grams (0.083mol) of dibromobiphenyl in a 500mL three-necked flask, 200ml of toluene, in 0.366 grams of cuprous iodide (1.92mmol), 4,4' - Under the catalysis of 0.299 grams of bipyridine (1.92mmol), react in the potassium hydroxide system for 8 hours to obtain the crude product through decolorization, washing, drying and distillation, then purify through the mixing of 80ml of toluene, 80ml of hexane, and 80ml of methanol, and finally obtain 20 grams of finished product ( Purity 97.8%), reaction yield 47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com