Method of growing myocardial cells
A cardiomyocyte and cell technology, applied in animal cells, vertebrate cells, genetically modified cells, etc., can solve problems such as unclear ubiquitin-protease system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] [Example 1: Preparation of recombinant adenovirus]
[0243] The adenoviral vector containing the CDK4 gene, the cyclin D1 (D1NLS) gene added with the nucleotide sequence encoding the nuclear localization signal (NLS), or the Skp2 gene was prepared using a recombinant adenovirus production kit (Adenovirus Expression Vector Kit; Takara Bio) make.
[0244] That is, when preparing Ad-CDK4, an adenoviral vector containing the CDK4 gene, plasmid pCMV-CDK4 (obtained from Dr. Sander van den Heuvel [Massachusetts General Hospital Cancer Center; USA]; van den Heuvel et al., Science 262: 2050, 1993) was cut with BamHI to prepare mouse CDK4 cDNA fragment, and after using T4 DNA polymerase to blunt the end, it was inserted into the SwaI site of cosmid pAxCAwt according to the operation instructions attached to the above recombinant adenovirus production kit to make cosmid pAd -CDK4. Then, recombinant adenovirus Ad-CDK4 was produced by transfecting the cosmid and restriction enzyme...
Embodiment 2
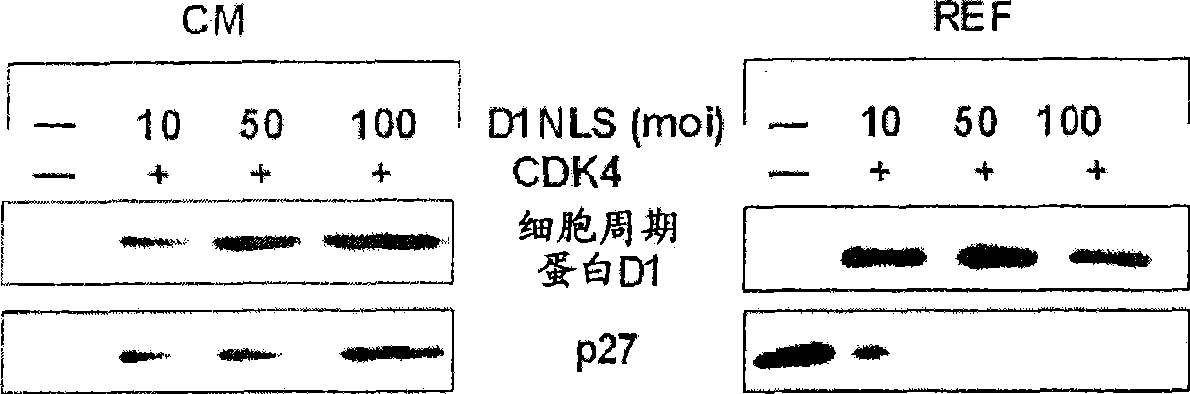
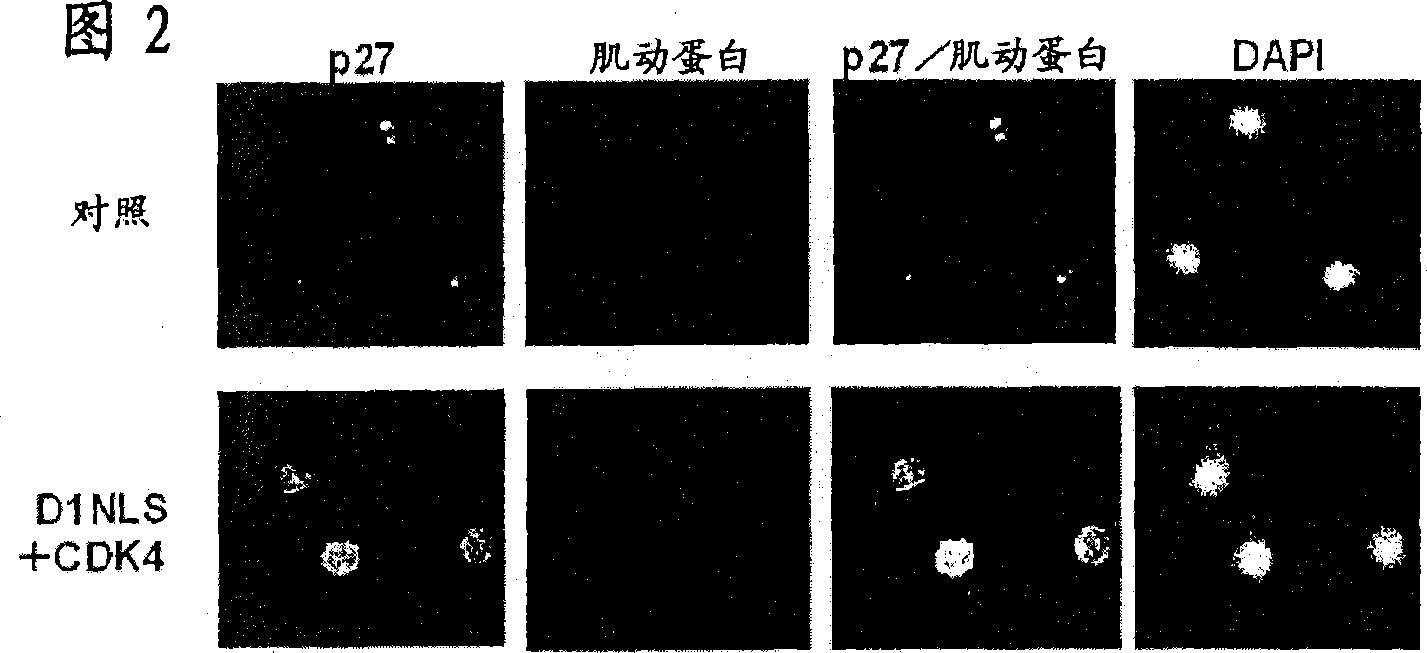
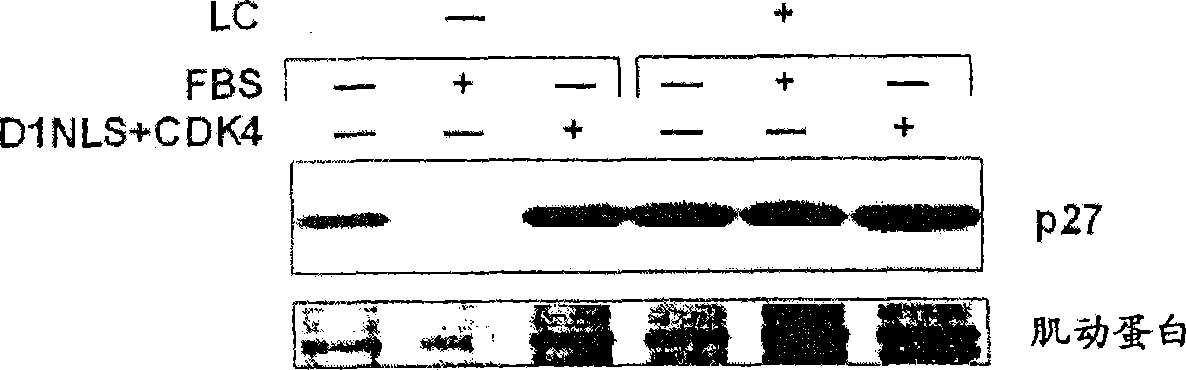
[0256] [Example 2: p27 Kip1 Protein accumulation in DNLS / CDK-treated cardiomyocytes]
[0257] Cardiomyocytes were isolated from 2-4 day old rats (Sprague-Dawley strain), and the cardiomyocyte fraction was recovered by Percol concentration gradient centrifugation (Tamamori et al., Am. J. Physiol. 275: H2036, 1998). 95% or more of the cells thus obtained were confirmed to be cardiomyocytes by immunostaining using an anti-sarcomeric actin antibody. The neonatal rat cardiomyocytes were suspended in Eagle basal medium (Flow Laboratories) supplemented with 5% fetal bovine serum (FBS; Flow Laboratories), inoculated on a culture dish, and cultured in a carbon dioxide incubator at 37°C 24 hours. The next day, the culture solution was replaced with serum-free Eagle basal medium, and after cultivating for 24 hours, the recombinant virus Ad-D1NLS (moi=10~100) and Ad-CDK4 (moi=100) prepared in Example 1 were Added to the culture medium and cultured for 48 hours. Hereinafter, the recomb...
Embodiment 3
[0268] 〔Example 3: The forced expression of Skp2 leads to the accumulation of p27 in cardiomyocytes Kip1 protein reduction]
[0269] It is known that in normal proliferating cells, p27 Kip1 Protein is contained as F-box protein Skp2 ubiquitin ligase or SCF Skp2 The complex is ubiquitinated and broken down by the proteasome (Carrano et al., Nature Cell Biol. 1:193, 1999; Tsvetkov et al., Curr. Biol. 9:661, 1999). Therefore, the expression of Skp2 protein in cardiomyocytes was investigated by immunoblotting. The preparation of cardiomyocytes, the transfection method of Ad-D1NLS and Ad-CDK4, the preparation of nuclear protein, and Western blot analysis were the same as the above methods, and the above-mentioned anti-Skp2 antibody was used to detect the Skp2 protein.
[0270] The result is as Figure 5shown. In fibroblasts (REF52 cells) used as control cells, the expression of Skp2 protein was significantly increased by FBS stimulation and D1NLS+CDK4 stimulation to promote pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com