Method of inducing antitumor immunity and its application in preparing medicine
An anti-tumor immunity and cell induction technology, applied in the field of biomedicine, to prevent and inhibit the growth of myeloma, improve the immune system, and improve the survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Low-dose live myeloma FO cells induce anti-tumor immunity
[0084] 1. Vaccine preparation:
[0085] 1.1 Dendritic cells derived from splenocytes: The erythrocytes in the mouse spleen were lysed with 8.3 g / ml ammonium chloride solution dissolved in 0.01M Tris-HCl. Unlysed spleen cells were cultured in serum-free RPMI1640 containing 2 mM glutamine, 2 mg / ml sodium bicarbonate, 25 mM HEPEs, 100 U / ml penicillin and 100 μg / ml streptomycin. at 5% CO 2 After incubation for 1 hour in the incubator, non-adherent cells were washed away. Adherent spleen cells continued to be treated with 10% fetal bovine serum, 2mM glutamine, 2mg / ml sodium bicarbonate, 25mM HEPEs, 10ng / ml IL-4 (PeproTech, UK), 10ng / ml GM-CSF (PeproTech, UK), 100U / ml penicillin and 100μg / ml streptomycin medium, and differentiated into dendritic cells. After 4 days in culture, adherent and loosely adherent dendritic cells were harvested by gentle pipetting, and the cell surface exhibited typical dendri...
Embodiment 2
[0105] Example 2: Myeloma FO cells transfected with GM-CSF induce anti-tumor immunity
[0106] 1. Vaccine preparation:
[0107] 1.1 Transfection of recombinant GM-CSF adenovirus into myeloma FO cells: Incubate cells with recombinant GM-CSF adenovirus suspension diluted 10 times with serum-free RPMI-1640. After culturing at 37°C for 1 hour, calf serum and serum-free RPMI-1640 were added to adjust the serum concentration to 10%, and incubated in an incubator for 24 hours.
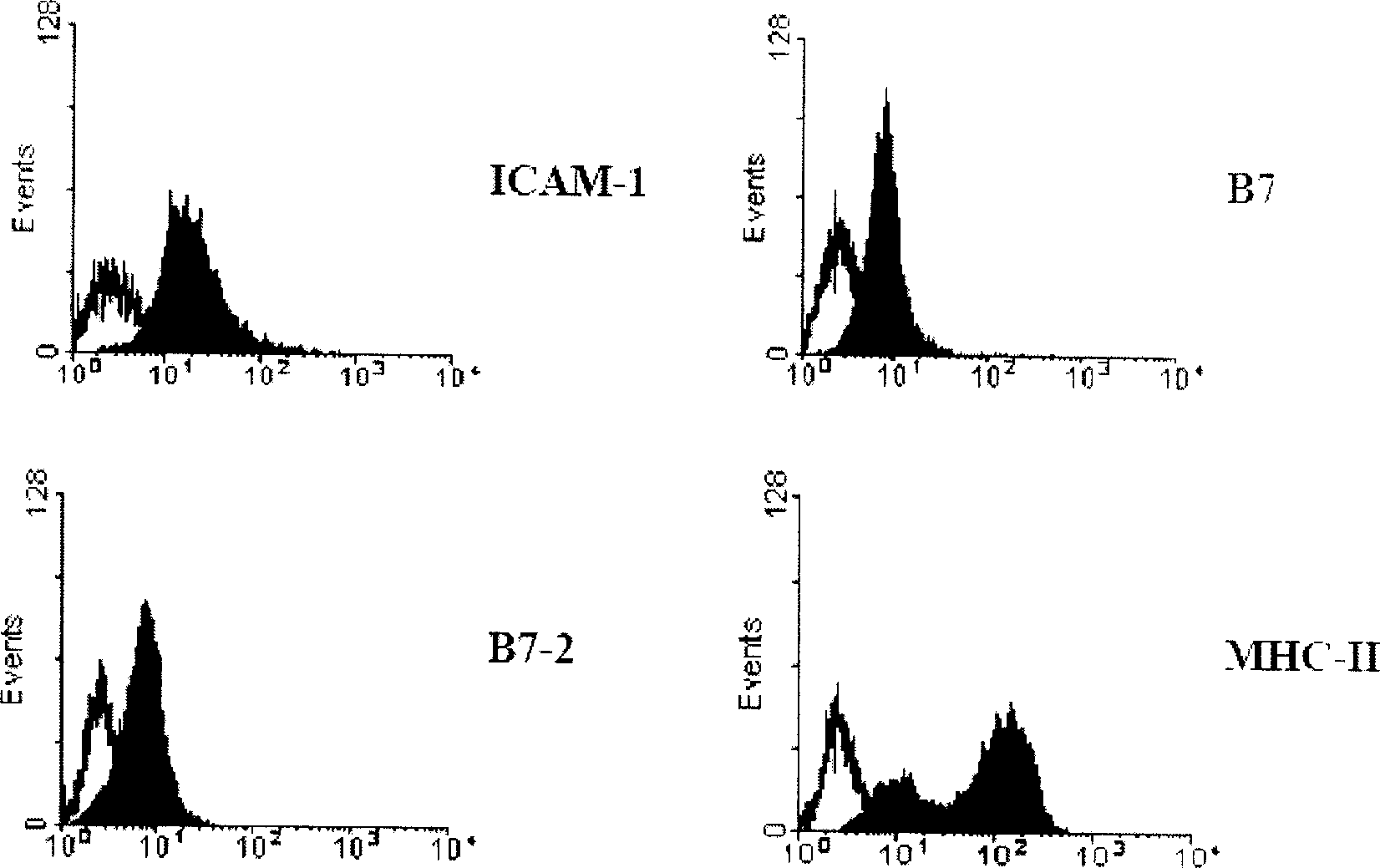
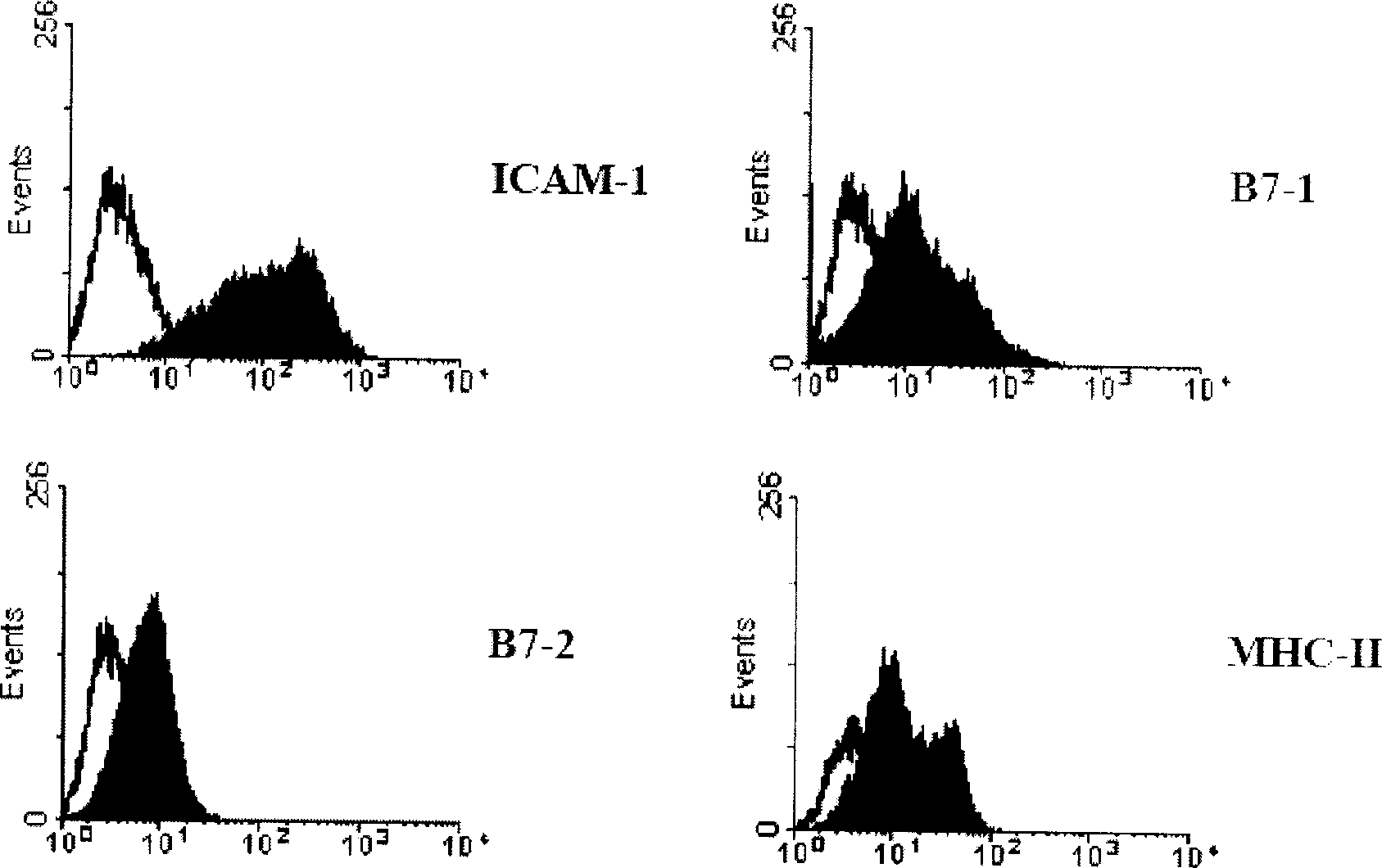
[0108] 1.2 Determination of transfection efficiency and GM-CSF secretion: collect transfected and non-transfected myeloma FO cells, fix with 2% (v / v) paraformaldehyde for 15 minutes, and then wash with 0.5% (v / v) paraformaldehyde ) Triton X-100 and 0.5% (w / v) BSA in PBS to permeate the cell membrane. Anti-mouse GM-CSF antibody (0.5 μg / 10 6 cells) were stained for 1 hour at room temperature and observed under a fluorescence microscope. Depend on Figure 4 It can be seen that more than 90% of the myeloma F...
Embodiment 3
[0116] Example 3: Live primary umbilical vein endothelial cells induce anti-tumor immunity
[0117] 1. Vaccine preparation:
[0118] 1.1 Preparation of primary human vascular endothelial cells: We prepared according to the method of separating vascular endothelial cells by Jaffe EA et al. [43] . Fresh human umbilical vein vessels were digested with type II collagenase, and the obtained human umbilical vein endothelial cells were cultured in 1% gelatin-coated culture dishes with IMDM medium containing 10% FBS.
[0119] 2. Evaluation of the anti-tumor effect of the vaccine:
[0120] 2.1 Live cell preventive immune anti-tumor model:
[0121] Mouse myeloma FO model: Primary human umbilical vein endothelial cells were harvested and resuspended in serum-free RPMI1640 medium. Female BALB / c mice were treated with 10 6 Immunization with primary human umbilical vein endothelial cells. Vaccine once a week for a total of four times. Inoculate subcutaneously 10 weeks after the last ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com