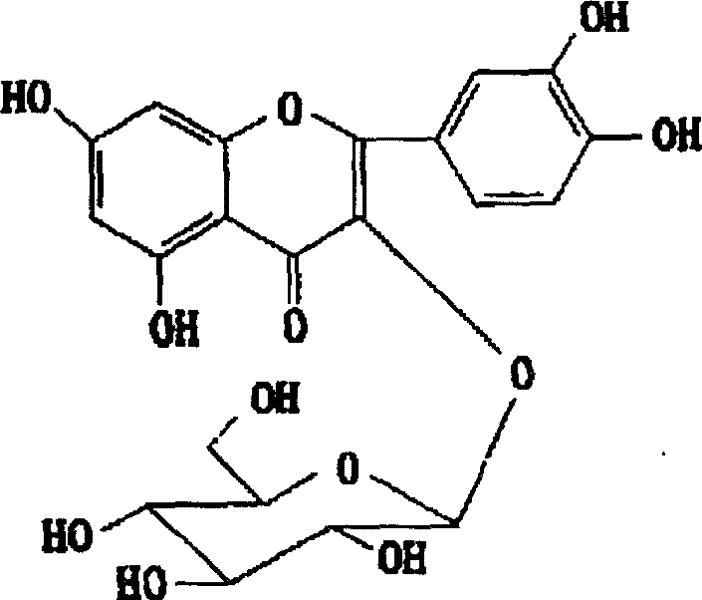
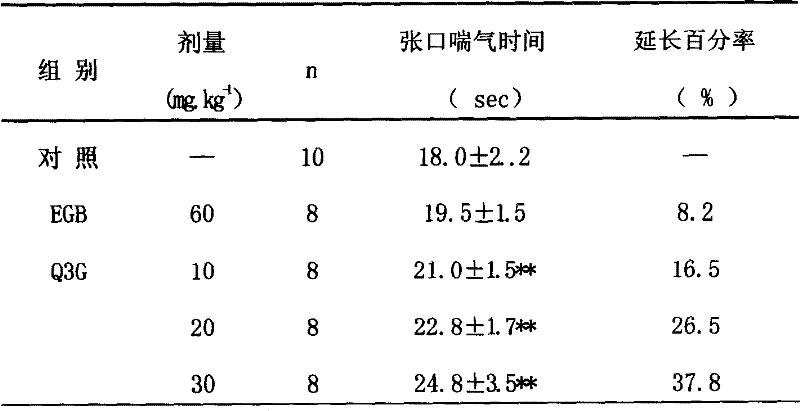
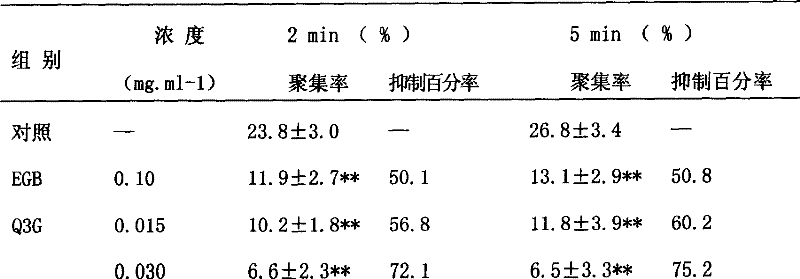
Medicinal use of quercetin-3-0-beta-D-glucancoside
A technology of glucoside and quercetin, which is applied in the field of new medical uses of known substances, can solve problems such as unseen cardiovascular and cerebrovascular diseases, and achieve the effects of reliable curative effect, high extraction cost and low extraction cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Synthesis of quercetin-3-O-β-D-glucoside
[0061] 1. Preparation of 7,3',4'-tri-O-benzyl quercetin
[0062] Rutin was dried under vacuum at 100°C for 6 hours to remove three crystal waters. Under the protection of N2, the dried rutin (2.44g) was dissolved in 20ml of anhydrous DMF, 1.83g of K2CO3 was added, after stirring for 1h, 1.6ml of freshly steamed benzyl bromide was slowly added dropwise, and then reacted at 40°C for 3h. The pH of the reaction solution was adjusted to 6 with 10% acetic acid in an ice bath, then 30 ml of distilled water was added thereto, the supernatant was discarded by centrifugation, and benzylated rutin was precipitated. Add 60ml of ethanol and 9ml of hydrochloric acid to the precipitate, and slowly heat to 70°C to completely dissolve the solid. After 10 minutes, a precipitate begins to precipitate in the solution, and the reaction ends after 2 hours. The reaction solution was cooled in an ice bath, and the precipitate was filtered...
Embodiment 2
[0069] Example 2: Quercetin-3-O-β-D-glucoside lyophilized powder for injection
[0070] Preparation of powder injection preparation (1):
[0071] Component Quantity
[0072] Quercetin-3-O-β-D-glucoside 5-50g
[0073] Tween 80 120g
[0074] Activated carbon 4g
[0075] Mannitol 400g
[0076] Poloxamer 8g
[0077] Quercetin-3-O-β-D-glucoside dry powder, Tween, poloxamer, melt it into a mortar and grind it to make it fully dispersed, add fresh water for injection saturated with carbon dioxide to 3500ml, add manna For alcohol, add activated carbon for needles in a water bath (50-70°C), stir while adding, boil for 5-10 minutes, cool to 40°C and filter through a vertical fusing filter. Dilute it with fresh water for injection to 4000ml, measure the pH and pass the content, pass through a 0.45um membrane, then pass through a 0.22um membrane, fill in 1000 vials, pre-freeze, and freeze to obtain 5-50mg of quercetin per bottle -3-O-β-D-glucoside lyophilized powder.
[0078] Prep...
Embodiment 3
[0079] Embodiment 3: quercetin-3-O-β-D-glucoside injection
[0080] Preparation of injection preparations (1)
[0081] Component Quantity
[0082] Quercetin-3-O-β-D-glucoside 5-50g
[0083] Sodium bicarbonate 22g
[0084] Sodium Metabisulfite 2g
[0085] Water for injection 1000ml
[0086] Take 1000ml of water for injection, boil, cool to room temperature, and set aside. Take 20% water for injection according to the prescription, add quercetin-3-O-β-D-glucoside to dissolve, slowly add sodium bicarbonate in stages and keep stirring, when there are no bubbles on the liquid surface, add coke Dissolve sodium sulfite, add the remaining water for injection to the full amount. Use a pH meter to measure the pH value of the medicinal solution and it should be 5.5-8.0. If it is not within this range, it can be adjusted with sodium bicarbonate. The medicinal liquid is filtered with a No. 3 vertical fusing glass filter until clear, filled with 500 ampoules, and sterilized at 100°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com