Improved gastrin releasing peptide compounds
A compound, targeting peptide technology, applied in the field of neogastrin-releasing peptide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0356] The following examples are provided as examples of different methods that can be used to prepare various compounds of the invention. In each example, there is a compound identified with a single bold capital letter (eg, A, B, C) that is related to a corresponding compound identically labeled in the figure.
[0357] General experiment
[0358] A. Definitions of other abbreviations used
[0359] The following common abbreviations are used throughout this specification:
[0360] 1,1-Dimethylethoxycarbonyl (Boc or Boc);
[0361] 9-fluorenylmethoxycarbonyl (Fmoc);
[0362] Allyloxycarbonyl (Aloc);
[0363] 1-Hydroxybenzotriazole (HOBt or HOBT);
[0364] N,N'-diisopropylcarbodiimide (DIC);
[0365] N-methylpyrrolidone (NMP);
[0366] Acetic anhydride (Ac 2 O);
[0367] (4,4-Dimethyl-2,6-dioxocyclohexylene-1-yl)-3-methylbutyl (iv-Dde);
[0368] Trifluoroacetic acid (TFA);
[0369] Reagent B (TFA:H 2 O: phenol: triisopropylsilane, 88:5:5:2);
[0...
Embodiment I-
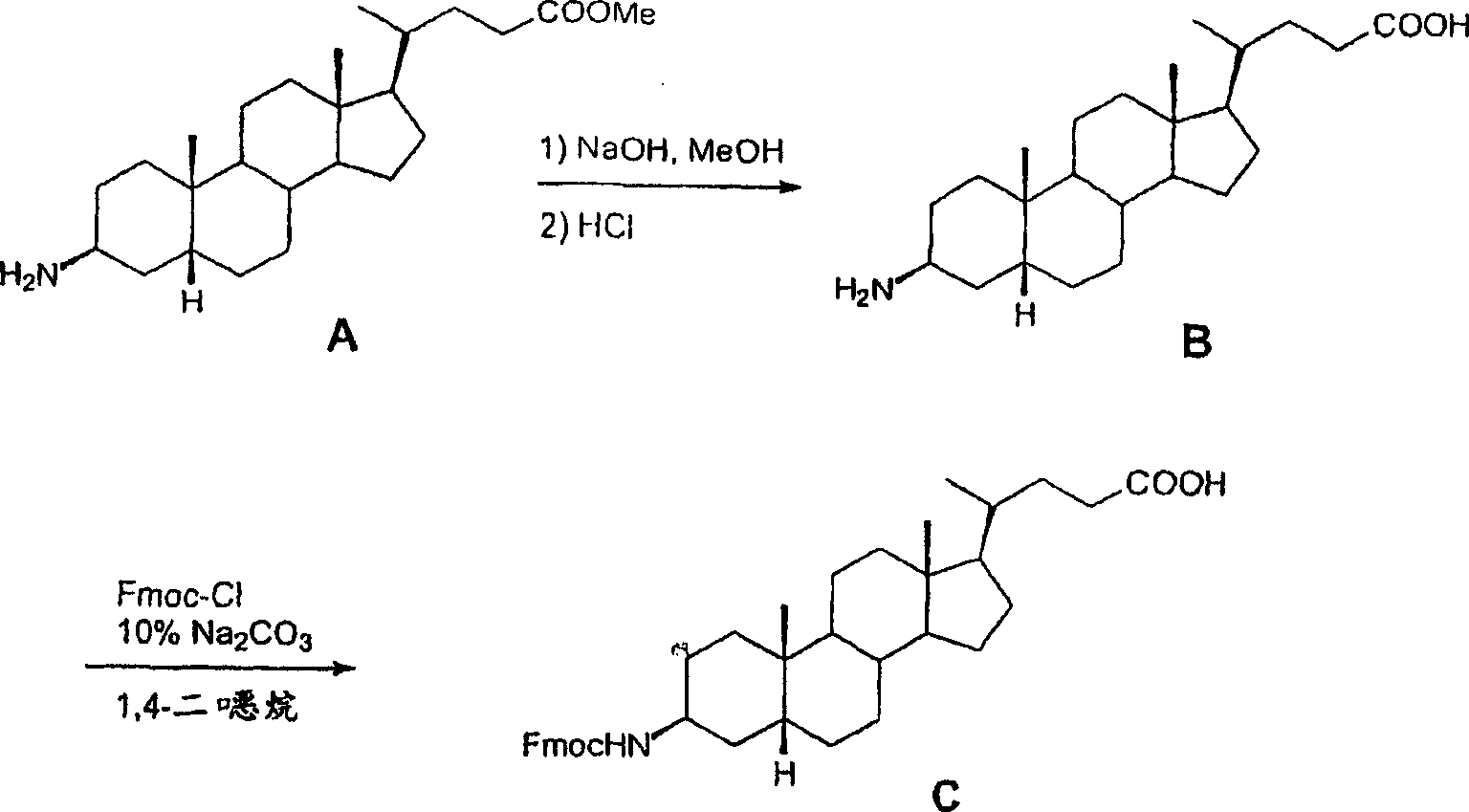
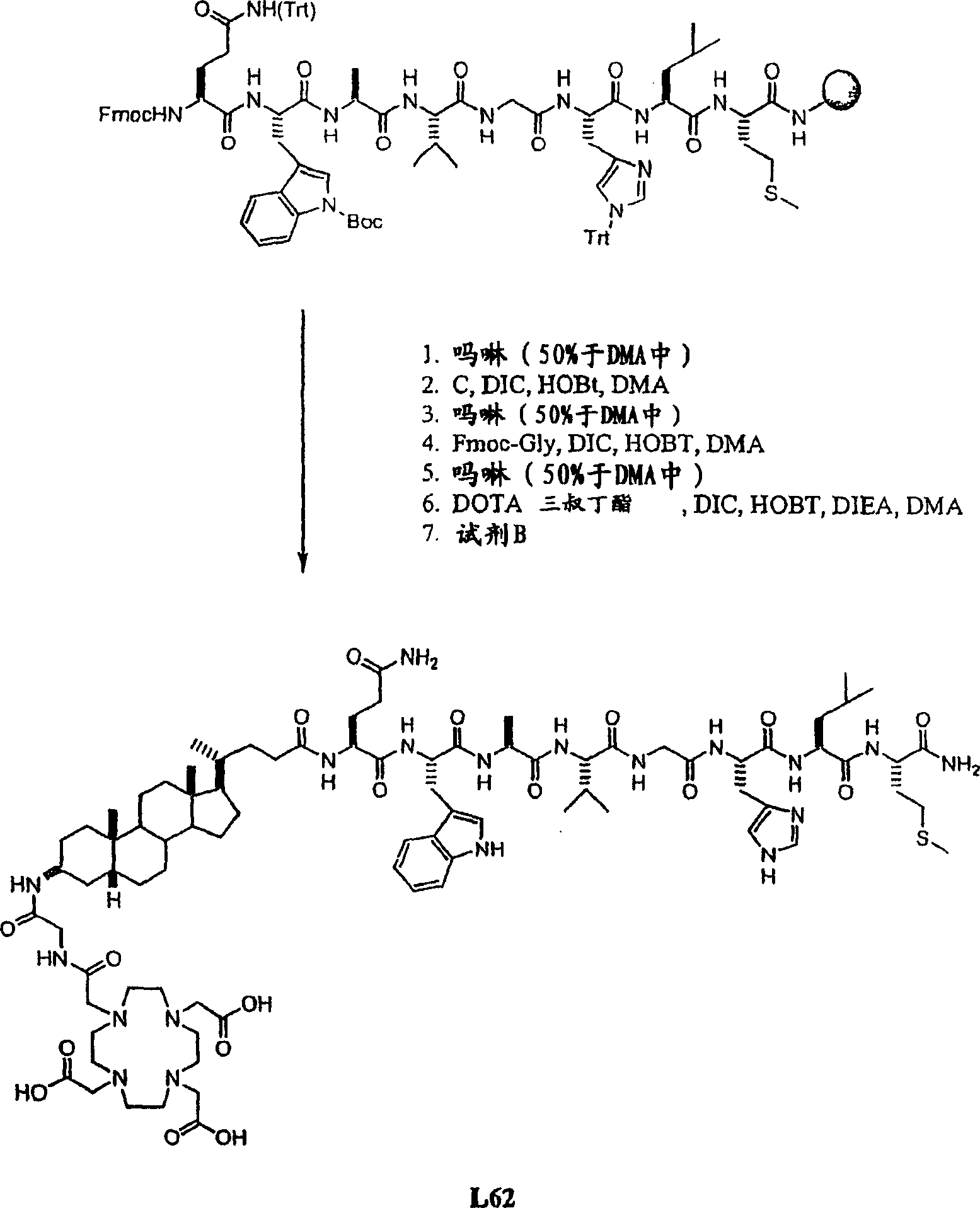
[0418] Example I- Figure 1A -B
[0419] Synthetic L62
[0420] Overview: as Figure 1A As shown in -B, prepare L62 with the following steps:
[0421] Hydrolysis of (3β,5β)-3-aminocholan-24-oic acid methyl ester A with NaOH gave the corresponding acid B, which was then reacted with Fmoc-Cl to give intermediate C. Using octapeptide Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH 2 (BBN[7-14][SEQ ID NO:1]) functionalized Rink amide resin was sequentially reacted with C, Fmoc-glycine and DOTA tri-tert-butyl ester. After cleavage and deprotection with Reagent B, the crude was purified by preparative HPLC to afford L62. Overall yield: 2.5%. More details are provided below:
[0422] A. Rink amide resin functionalized with bombesin [7-14], (A)
[0423] In a solid-phase peptide synthesis vessel (see Appendix No.1), combine Fmoc-amino acid (24mmol), N-hydroxybenzotriazole (HOBt) (3.67g; 24mmol) and N,N′-diisopropylcarbon Diimine (DIC) (3.75 mL; 24 ...
Embodiment II-
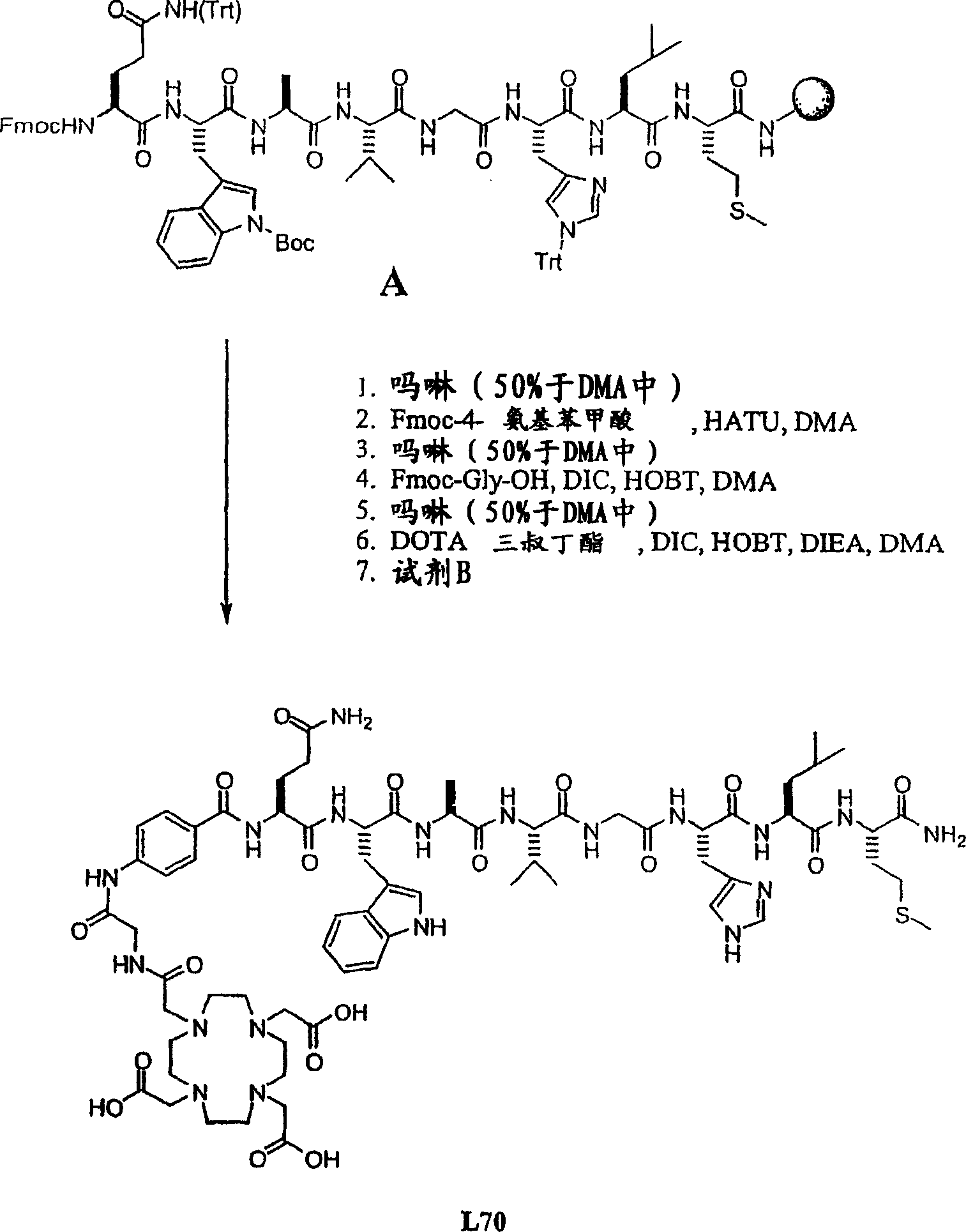
[0434] Example II- Figure 2A -F
[0435] Synthesis of L70, L73, L74, L115 and L116
[0436] Overview: The product is obtained by the following method: the octapeptide Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH loaded on the Rink amide resin 2 (BBN[7-14][SEQ ID NO: 1]) (with appropriate side chain protection) was coupled to different linkers and then functionalized with DOTA tri-tert-butyl ester. After cleavage and deprotection with reagent B, the final product was purified by preparative HPLC. Overall yield 3-9%.
[0437] A. Synthesis of L70 ( Figure 2A ):
[0438]Resin A (0.5 g; 0.3 mmol) was shaken with 50% morpholine in DMA (7 mL) in a solid phase peptide synthesis vessel for 10 minutes, the solution was emptied and fresh 50% morpholine in DMA (7 mL) was added. Morpholine. The suspension was stirred for 20 minutes, then the solution was emptied and the resin was washed with DMA (5 x 7 mL). Fmoc-4-aminobenzoic acid (0.43 g; 1.2 mmol), HOBT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com