Fe (II)-Nd (III) hexascrew nanometer pipe polymer and preparation method
A technology of tubular polymers and polymers, applied in organic chemistry and other fields, can solve problems such as large variation range, difficulty in selecting ligands, interpenetration of lattices, etc., and achieve high thermal stability, wide application prospects, and high thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 [Nd(PDA) 3 Fe 1.5 (H 2 O) 3 ]·2.5H 2 Synthesis of O:
[0021] 0.1 mmol Nd 2 o 3 (0.033g), 0.6mmol FeSO 4 ·7H 2 O (0.167g), 0.8mmol PDA (0.134g) mixture, put into the polytetrafluoroethylene liner of 20mL hydrothermal reaction kettle, first add 4mL CH 3 CN, followed by the rapid addition of 8mL H 2 O, in case of Fe 2+ After being oxidized and sealed tightly with a stainless steel sleeve, the temperature was quickly raised to a high temperature of 150°C, and after a constant temperature of 72 hours, the temperature was programmed to cool down to room temperature. The obtained product was washed twice with 5mL water, and finally a deep red polyhedral prism suitable for single crystal diffraction was selected. shaped crystals. The calculated yield based on metallic Nd was 65%.
Embodiment 2
[0022] Embodiment 2 [Nd(PDA) 3 Fe 1.5 (H 2 O) 3 ]·2.5H 2 Characterization of O:
[0023] (1)[Nd(PDA) 3 Fe 1.5 (H 2 O) 3 ]·2.5H 2 Structure determination of O
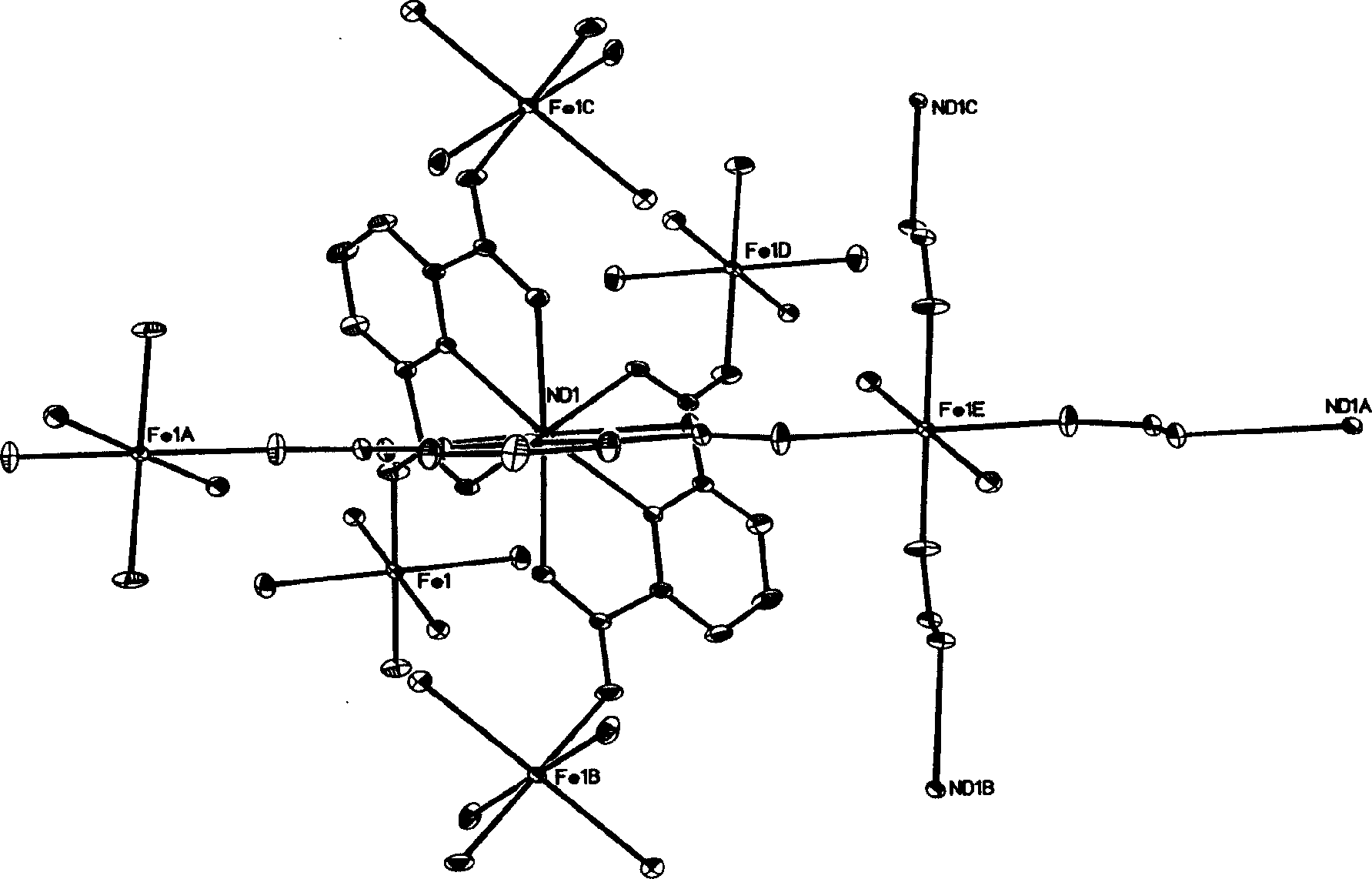
[0024] The crystal structure was determined by BRUKER SMART 1000 X-ray diffractometer, using graphite monochromatized Mokα rays (λ=0.71073 Ȧ) as the incident radiation, collecting diffraction points in ω-Φ scanning mode, and correcting the unit cell parameters by the least square method , the crystal structure was obtained from the difference Fourier electron density map using the SHELXL-97 direct method, and corrected by Lorentz and polarization effects. All H atoms were synthesized by difference Fourier and determined by ideal position calculation. The detailed crystal determination data are shown in Table 1. structure see figure 1 , figure 2 as well as image 3 ; figure 1 : [Nd(PDA) 3 Fe 1.5 (H 2 O) 3 ]·2.5H 2 Crystal structure diagram of the O unit. figure 2 : Six-helix structure constituting...
PUM
| Property | Measurement | Unit |
|---|---|---|
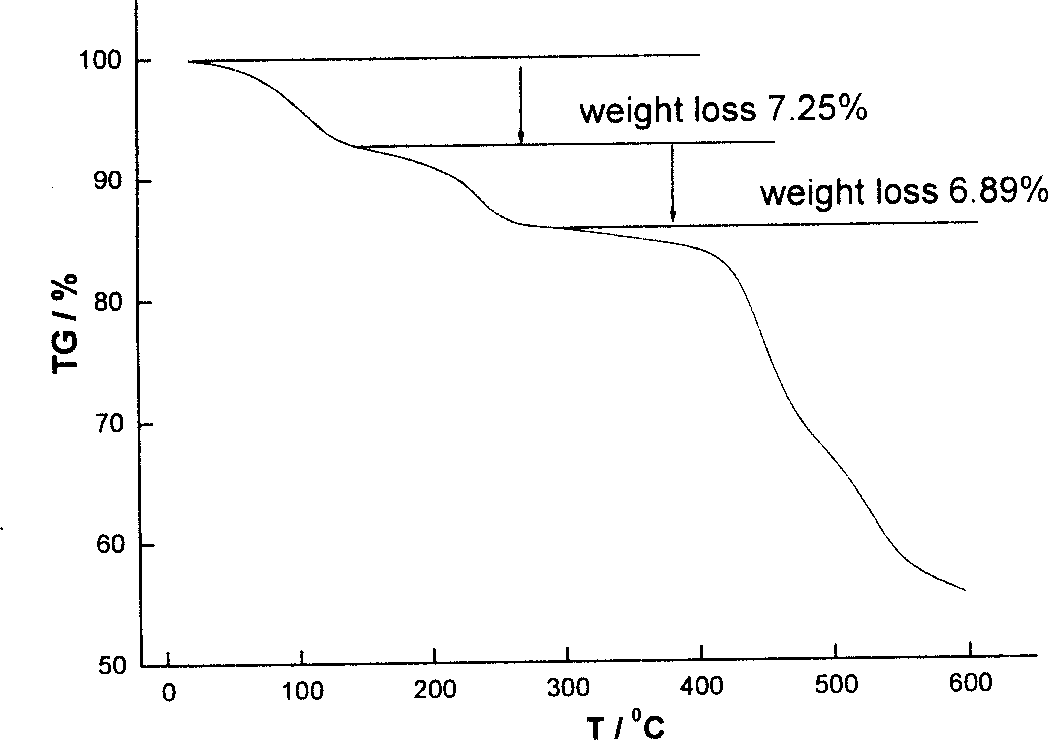
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com