Method for synthesizing methyl azulenoids
A technology of methyl ophthalmic compounds and a synthesis method, which is applied to the synthesis field of non-benzene aromatic compounds, can solve the problems of low yield, high price of organic imines, expensive reagents and the like, and achieves improved yield, improved yield, The effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
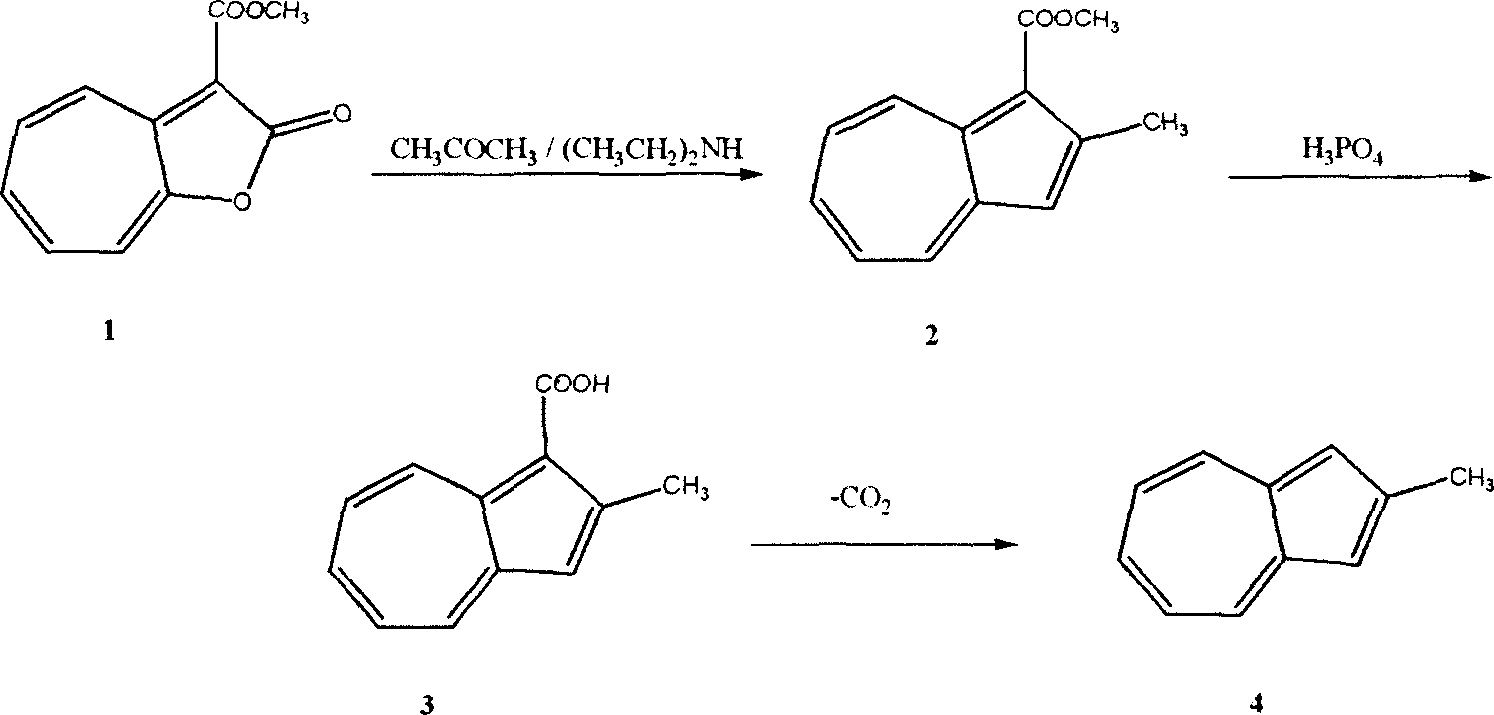
[0035] One, the synthesis of methyl 2-methyl azulene-1-carboxylate
[0036] In a 100ml three-necked reaction flask, molecular sieves (3A, 3.0 grams) were added to acetone (20 milliliters) containing cycloheptafuran-2-one-3-carboxylic acid methyl ester (1.0 grams, 5.0 mmol) and diethyl In the amine (7ml) solution, reflux for 24 hours, cool to room temperature, add water (50ml), extract and separate through benzene (3*20ml), wash with water, recover the solvent, and distill the residue under reduced pressure, collect 115~118℃ / 5mmHg fraction, 0.8 g of cyan oil was obtained, the yield was 79%, and solidified after standing. Melting point: 45-46°C.
[0037] Its structure is 2-methyl azulene-1-carboxylate methyl ester according to nuclear magnetic resonance analysis, and its purity as determined by liquid chromatography is 99.1%.
[0038] According to the above experimental process, the type of molecular sieve and the reaction time were changed, and other reaction conditions and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com