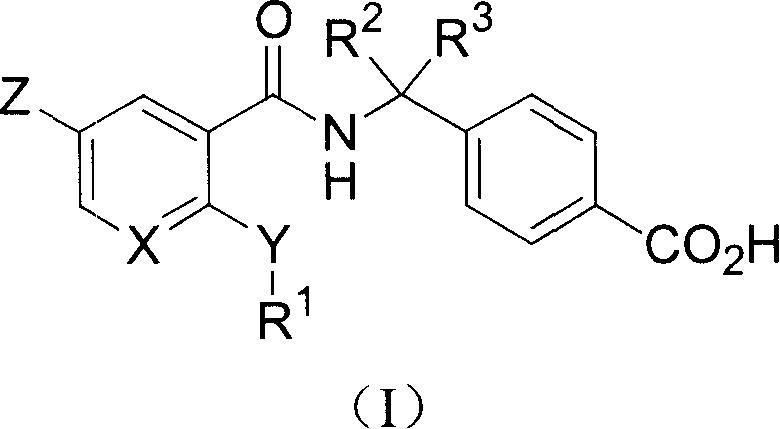
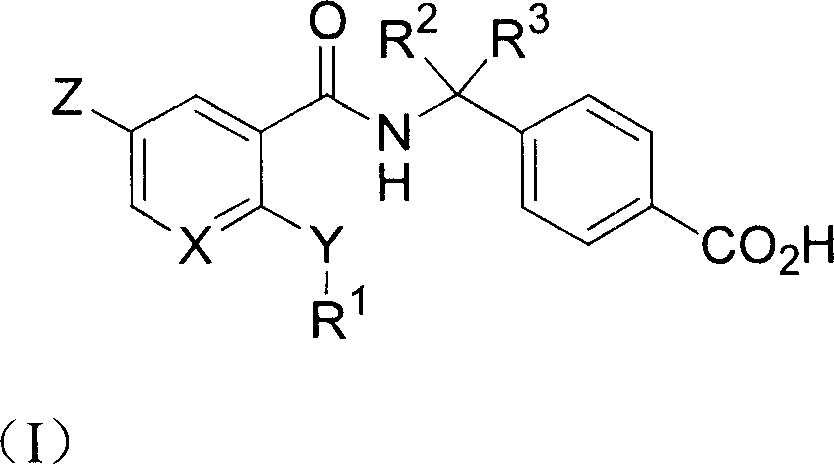
Ortho substituted aryl or heteroaryl amide compounds
A compound and heteroaryl technology, applied in the direction of active ingredients of heterocyclic compounds, preparation of organic compounds, drug combination, etc., to achieve the effect of improving half-life, low toxicity and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
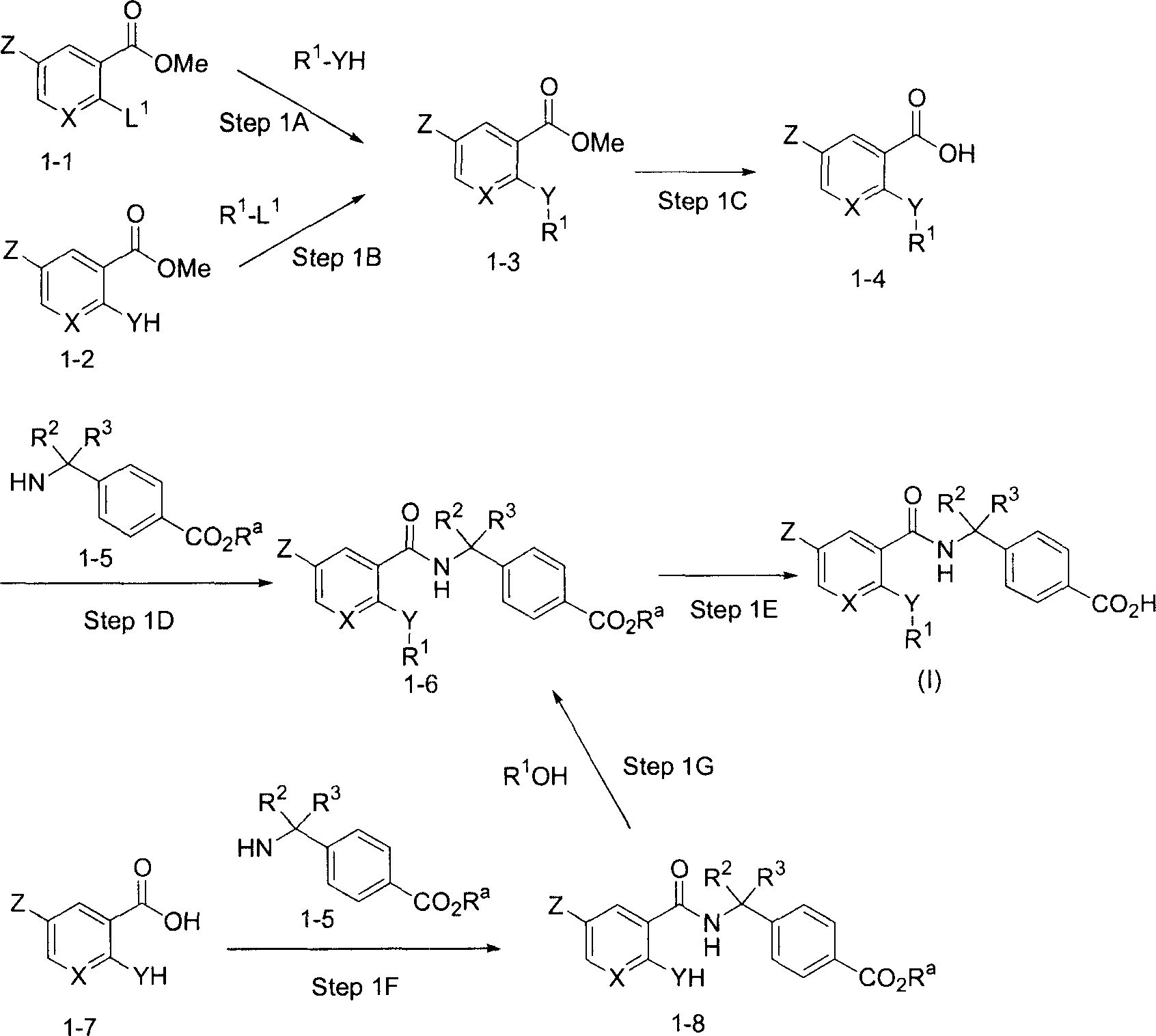
[0097] This scheme shows the preparation of compounds of formula (I).
[0098] Route 1
[0099]
[0100] In the above formula, R a represents an alkyl group having 1 to 4 carbon atoms. L 1 Indicates a leaving group. Examples of suitable leaving groups include: halogen atoms such as chlorine, bromine and iodine; sulfonates such as TfO (trifluorosulfonate), MsO (mesylate); or boronic acid groups.
[0101] Step 1A
[0102] In this step, through the ester compound of formula 1-1 and formula R 1 The compound of formula 1-3 can be prepared by the coupling reaction of the compound of -YH in an inert solvent.
[0103] The coupling reaction can be carried out in a reaction-inert solvent or without a solvent, in the absence or presence of a base. Preferred bases are selected from, for example, alkali metal or alkaline earth metal hydroxides, alkoxides, carbonates or hydrides, such as sodium hydroxide, potassium hydroxide, sodium methoxide, sodium ethoxide, potassium tert-butox...
Embodiment 1
[0310] 4-({[5-Chloro-2-(2-phenylethoxy)benzoyl]amino}methyl)benzoic acid
[0311]
[0312] MS (ESI) observed value: m / z 409.99 (M+H) +
[0313] C 23 h 20 ClNO 4 Exact mass calculated: m / z 409.11
Embodiment 2
[0315] 4-[({5-chloro-2-[2-(2-chlorophenyl)ethoxy]benzoyl}amino)methyl]benzoic acid
[0316]
[0317] MS (ESI) observed value: m / z 443.92 (M+H) +
[0318] C 23 h 19 Cl 2 NO 4 Exact mass calculated: m / z 443.07
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com