Compsn of anti-blood generating factor contg adeno-associated virus mediation and its application
An anti-angiogenesis and composition technology, applied in the direction of medical preparations containing active ingredients, viruses, microorganisms, etc., can solve the problems of unsatisfactory effect and short action time, and achieve enhanced anti-tumor effect, long action time, stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] 7. Preparation of HGFK1 protein antibody
[0075] Using standard techniques of preparation (Harlow, E., and Lane, D. (1988) Antiodies: a laboratory manual (antibodies: a laboratory manual). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory) or methods known in the art, can be The construct of the present invention or the protein expressed thereof is used to prepare antibodies against the protein of the present invention.
[0076]For example, the constructs of the invention can be purified to the extent necessary to immunize animals such as rabbits. To prevent potential problems with low affinity or low specificity of antisera, 2 or 3 constructs for anti-angiogenic factors can be prepared and each construct injected into at least 2 animals. Antiserum can be generated by a series of injections, preferably comprising at least three booster injections. The primary immunization can be performed with complete Freund's adjuvant, followed by booster immunization with inco...
Embodiment 1
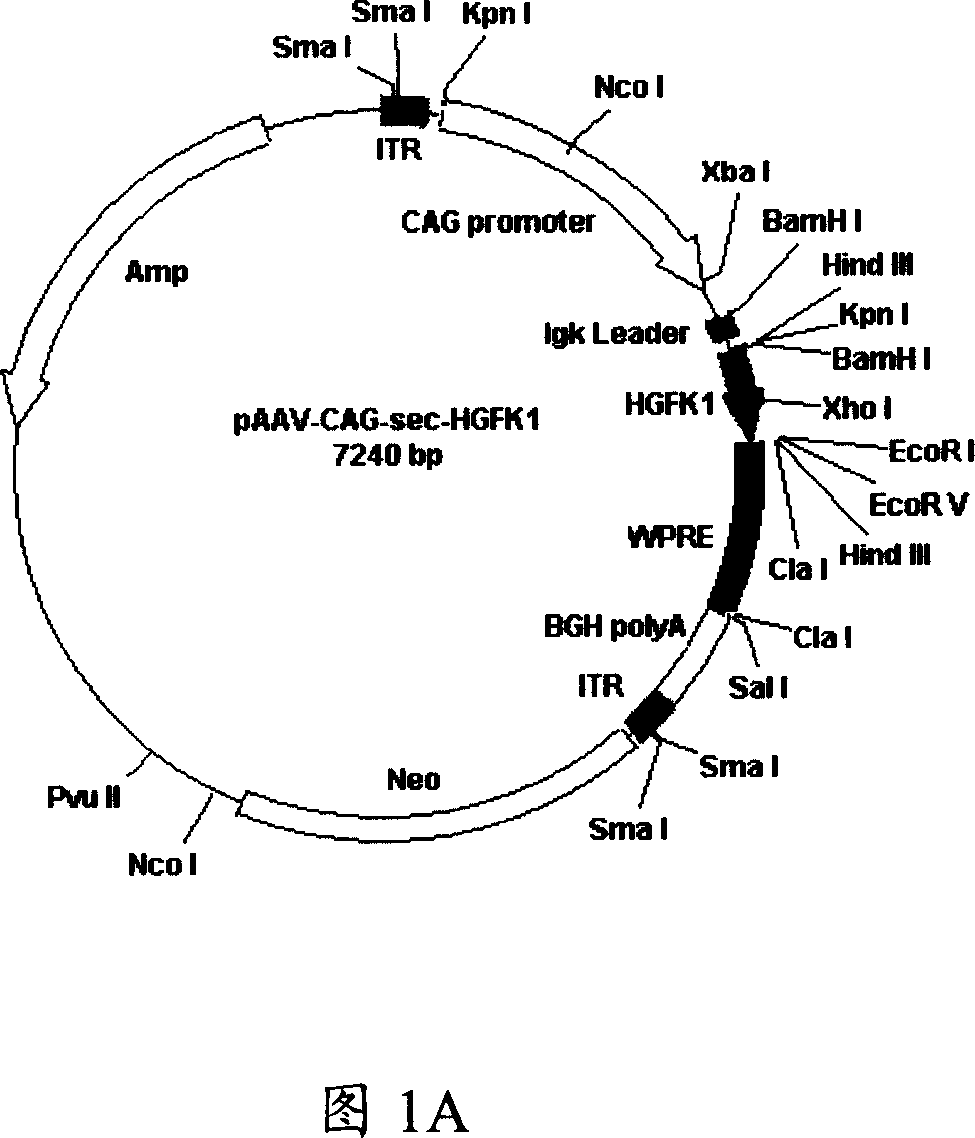
[0084] The construct of the AAV expression vector of embodiment 1 coding human HGFK1 polypeptide
[0085] The cDNA of human HGFK1 (SEQ ID NO: 1 nucleotide sequence) is inserted into the AAV packaging vector of pAM / CAG / EGR-1-pL-WPRE-BGH-polyA that has been digested with the same endonuclease in advance to form AAV - HGFK1 recombinant vector. Then use the three-vector helper virus defect packaging system (AAV, H22 and pFD6) for packaging, that is, use the calcium carbonate method to co-transfect HEK-293 cells with the recombinant vector AAV-HGFK1 and helper plasmids H22 and pFD6, and transfect for 60-72 hours Afterwards, the cells were harvested, the isolated recombinant virus particles were purified by HiTrap Heparin affinity chromatography, and the final titer was determined by real-time PCR.
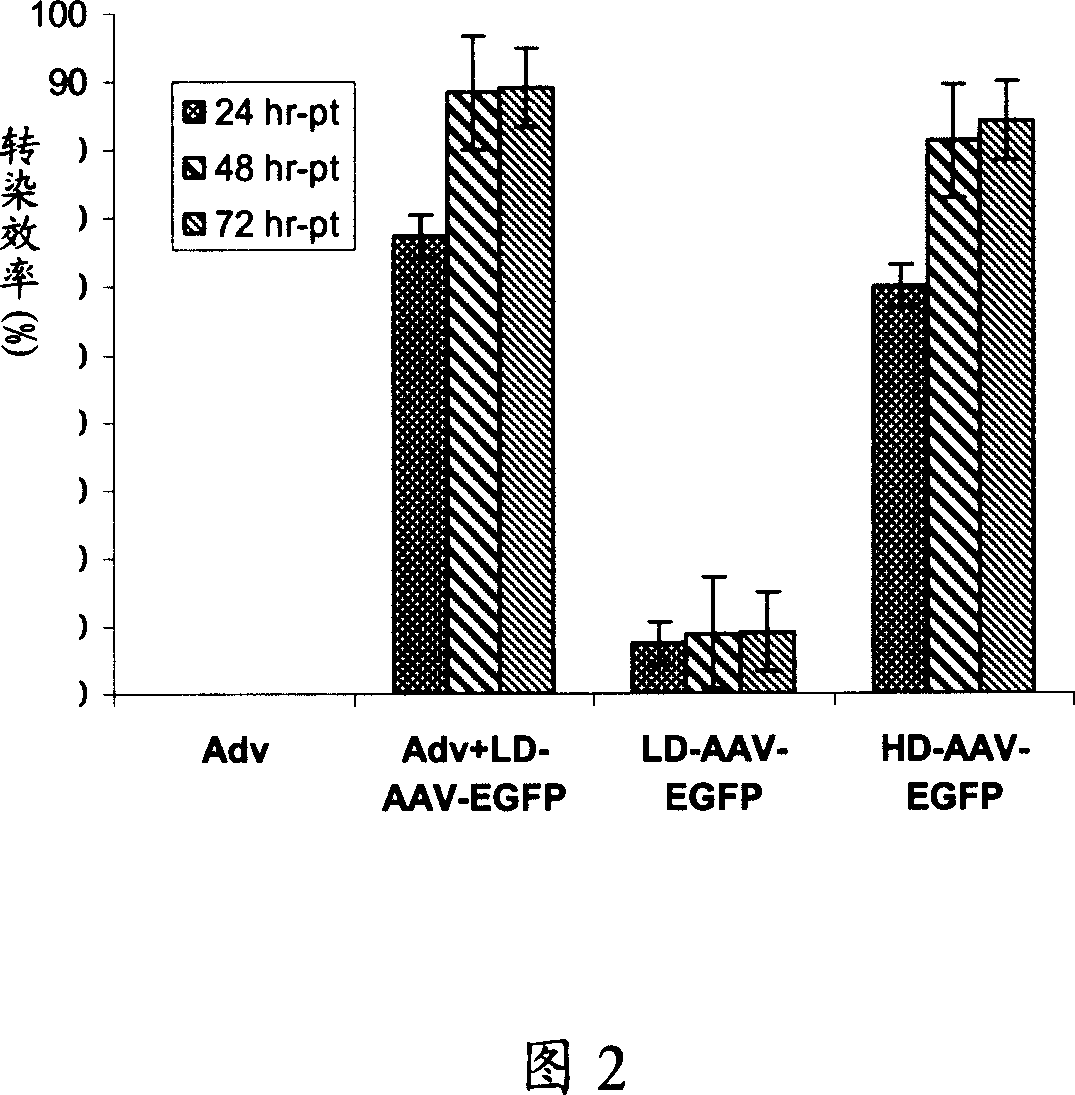
[0086] After infecting mouse microvascular endothelial cells and rat hepatocellular carcinoma cells with AAV-HGFK1 in vitro, the infected cells were stained by immunohistochemistry. T...
Embodiment 2
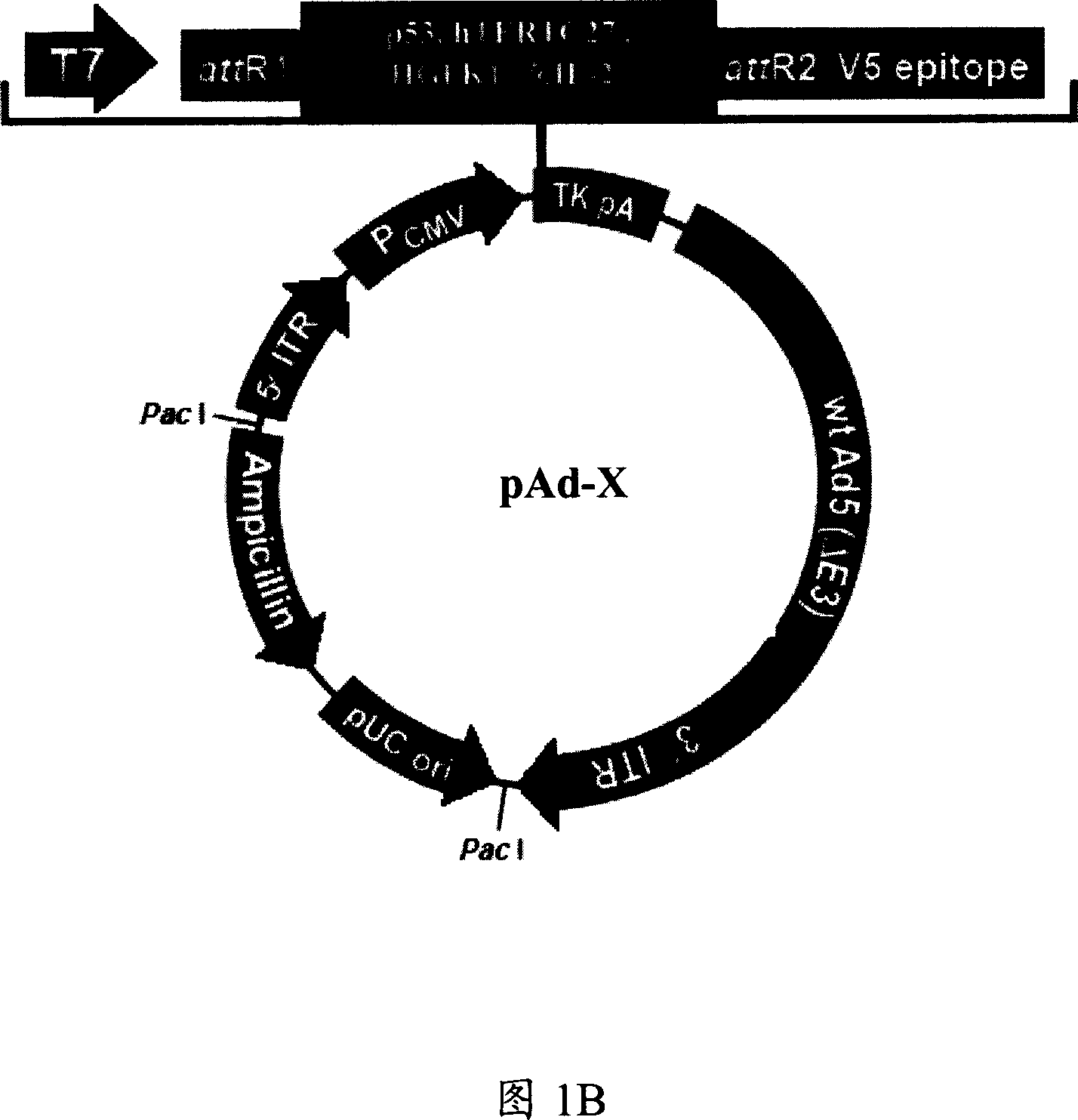
[0088] Embodiment 2 encodes p53, the construct pAd-X of the adenoviral vector (Adv) of hTERTC27, IL-2 or HGFK1 gene
[0089] The present invention provides the preparation and packaging method of the construct of recombinant defective adenovirus expression vector encoding human p53, hTERTC27, IL-2 or HGFK1 gene, comprising the following steps: human p53 gene, hTERTC27 gene, IL-2 gene or HGFK1 Genes were ligated into the shuttle plasmid pENTR TM In 2B, the recombinant shuttle plasmids pENTR-p53, pENTR-hTERTC27, pENTR-IL-2 or pENTR-HGFK 1 and pAd / CMV / V5-DEST were obtained by homologous recombination into expression vectors pAd-p53, pAd-hTERTC27, pAd-IL-2 or pAD-HGFK1, the resulting expression vector was treated with Lipofectamine TM The 293A cells were transfected with 2000 Reagent (Invitrogen), and the cells were lysed, concentrated and purified through a cesium chloride column. The purified recombinant adenovirus (Adv-p53, Adv-hTERTC27, Adv-IL-2 or Adv-HGFK1) carrying human...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com