Chemical synthesizer capable of preparing multiple fluoro-marking agent
A technology of chemical synthesis and reagents, applied in chemical instruments and methods, chemical/physical processes, radioactive preparations in vivo, etc., can solve problems such as high production costs, lack of chemical synthesis equipment, and inseparable steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]This device separates and purifies to obtain nucleophilic reaction 18 f - ion.
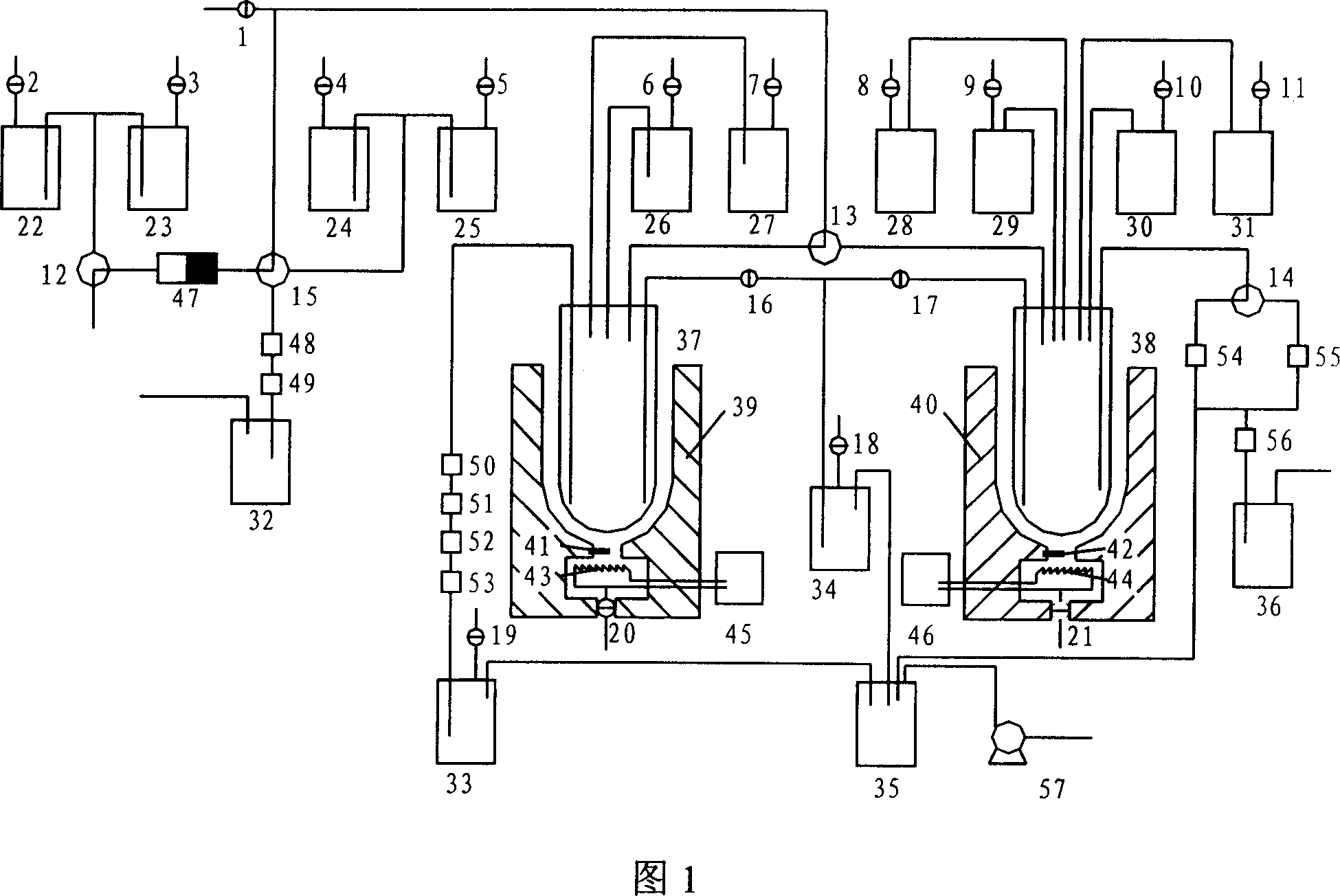
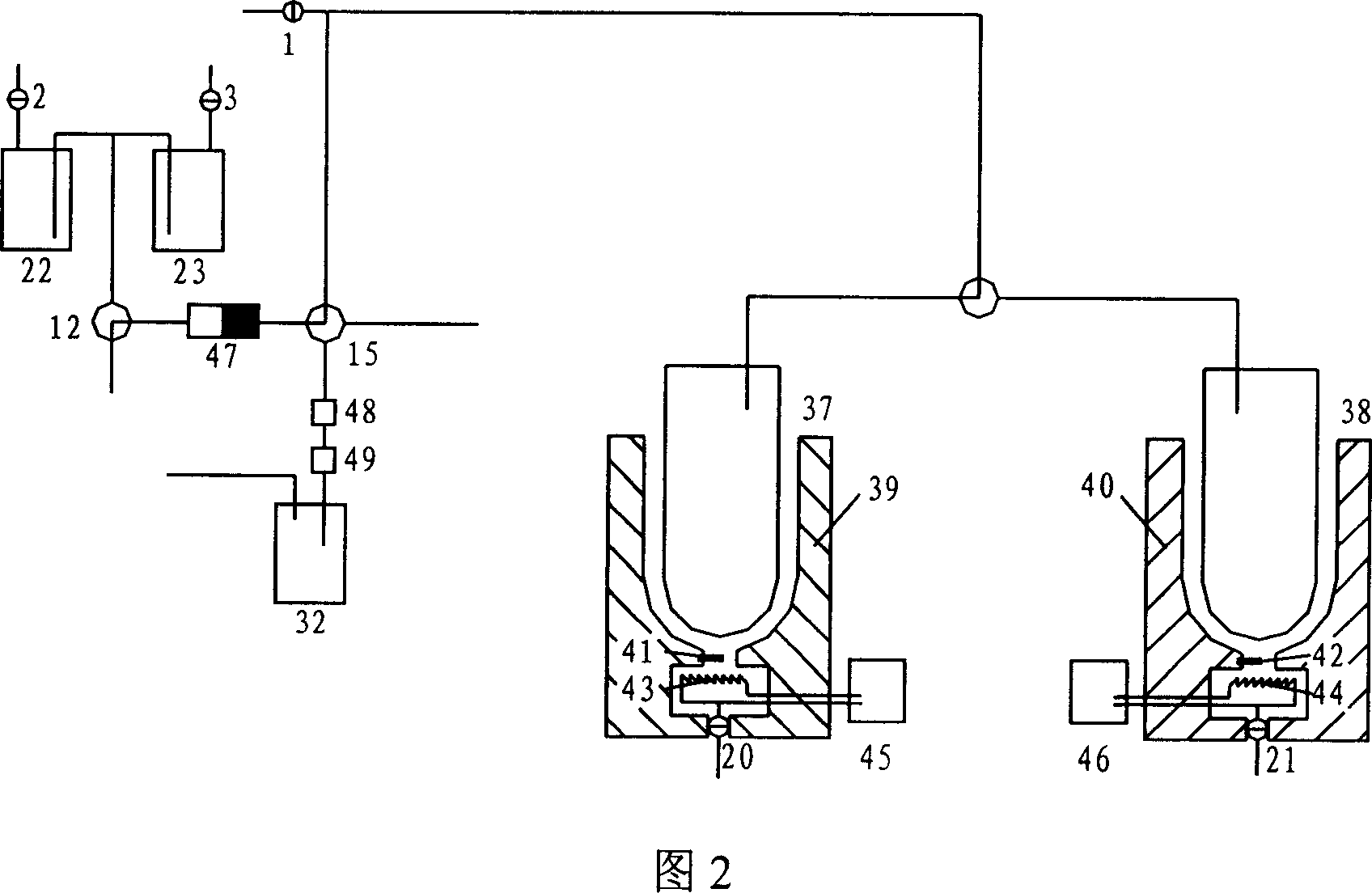
[0025] As shown in Figure 2, the preparation of separation and purification 18 f - Ions, a schematic diagram of the process structure for recovering oxygen-18 raw materials. First, prepare K with potassium carbonate 2.22 Anhydrous acetonitrile solution, it is added in the reagent bottle 22, anhydrous acetonitrile is added in the reagent bottle 23, open accelerator and carry out 18 O(p,n) 18 F reaction, then just can carry out preparation operation, concrete operation steps are as follows:
[0026] 1. Pass by the accelerator 18 O(p,n) 18 F reaction generation 18 F, on-target 18 F is transmitted by argon, through the three-way solenoid valve 12, 18 f - The ions are captured by the anion column QMA47, and the unreacted oxygen-18 raw material is purified after passing through the IC-H column 48 and IC-OH column 49 to remove the heavy metal ions from the metal target, and enters the o...
Embodiment 2
[0031] Prepared using this device 18 F-FDG.
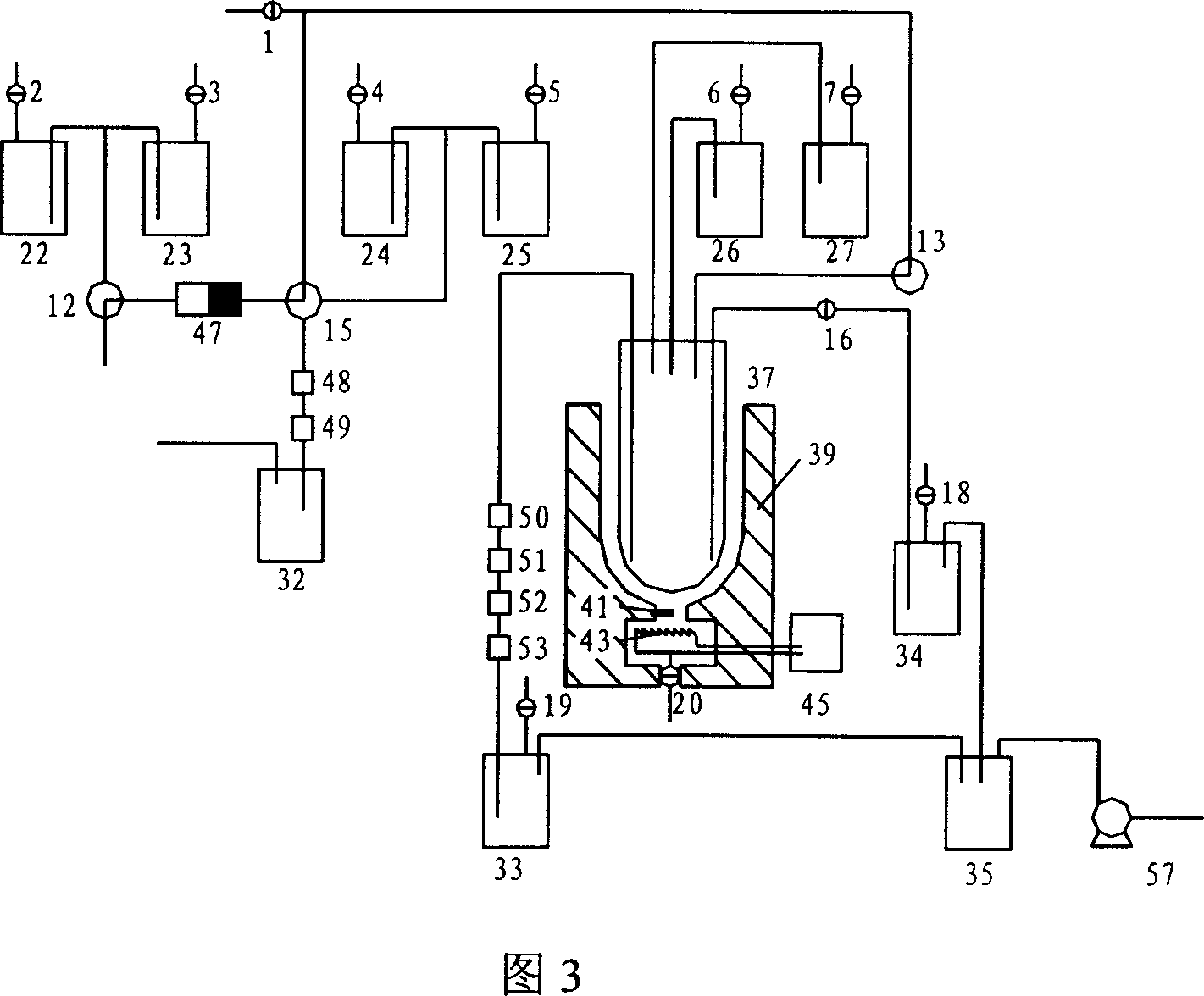
[0032] As shown in Figure 3, the preparation 18 Schematic diagram of the flow structure of F-FDG. First, the K containing potassium carbonate 2.2.2 Add anhydrous acetonitrile solution to reagent bottle 22, add anhydrous acetonitrile to reagent bottle 23, add dilute alkali solution (0.3N NaOH or KOH) to reagent bottle 24, add dilute acid (1N HCl) to reagent bottle In 25, mannose trifluoride dissolved in anhydrous acetonitrile is added in the reagent bottle 26, pure water is added in the reagent bottle 27, and the accelerator is turned on to carry out 18 O(p,n) 18 F reaction, then just can carry out preparation operation, concrete operation steps are as follows:
[0033] 1. according to the step 1,2,3 of embodiment 1, will isolate and purify 18 f - Ions are transferred to the tubular reactor 37;
[0034] 2. Open the two-way solenoid valve 20, open the thermostat 45, adjust the temperature of the resistance wire 43 through the...
Embodiment 3
[0045] Prepared using this device 18 F-MPPF.
[0046] As shown in Figure 4, the preparation 18 Schematic diagram of the flow structure of F-MPPF. First, the K containing potassium carbonate 2.2.2 Add anhydrous acetonitrile solution to reagent bottle 22, add anhydrous acetonitrile to reagent bottle 23, add dilute alkali solution (0.3N NaOH or KOH) to reagent bottle 24, add dilute acid (1N HCl) to reagent bottle In 25, 4-(2'-methoxy-phenyl)-1-[2'-(n-2"-pyridyl)-p-nitrobenzamide]-ethylpiperazine (MPPNO 2 ) dissolved in anhydrous dimethyl sulfoxide (DMSO) was added to reagent bottle 28, anhydrous ether was added to reagent bottle 29, methanol / tetrahydrofuran (THF) mixed solution was added to reagent bottle 30, and HPLC washing liquid was added to In the reagent bottle 31, open the accelerator to carry out 18 O(p,n) 18 F reaction, then just can carry out preparation operation, concrete operation steps are as follows:
[0047] 1. according to the step 1,2,3 of embodiment 1, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com