Guanine one-pot synthesis method
A synthesis method and guanine technology are applied in the field of one-pot synthesis of guanine, can solve problems such as long synthesis routes, and achieve the effects of convenient operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
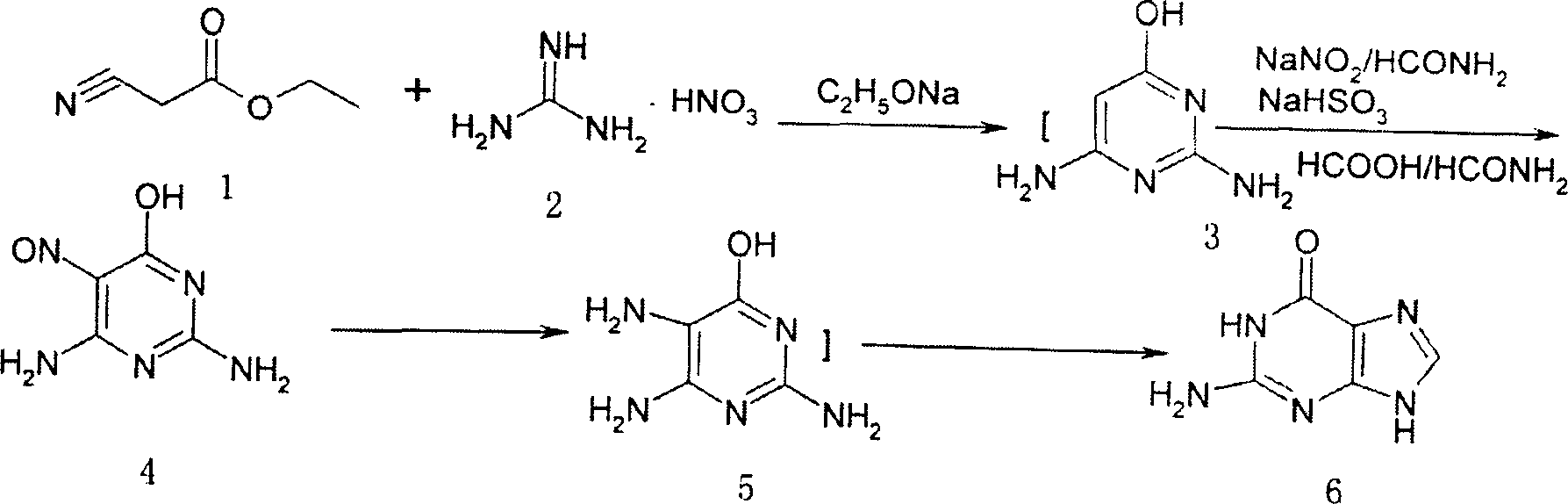
[0026] Add 400mL of absolute ethanol and 18.0g (0.783mol) of sodium metal into a 2L four-neck flask, stir, and add 110.0g (0.898mol) of guanidine nitrate after it is completely dissolved, heat to 70°C and stir for 0.8 hours. 56.0 g (0.486 mol) of ethyl cyanoacetate was added dropwise, heated to 70° C. (range 50° C. to reflux) and reacted for 2 hours, and the ethanol was evaporated to dryness under reduced pressure. Add 600ml of formamide to dissolve, and cool to 4°C in ice water. Add 40.0 g (0.580 mol) of sodium nitrite, add dropwise a mixed solution of 100 ml formamide and 100 ml 98% formic acid, and control the temperature below 5°C. After dropping, react at 4°C for 2.5 hours. Heat to 100°C, add 20.0 g (0.192 mol) of sodium bisulfite, and react at 100°C for 1.5 hours. Reaction was carried out at 180° C. for another 2 hours. Cool, filter and wash with water several times. After drying, 147g of brown solid was obtained.
[0027] Add 2000 mL of 2.75N hydrochloric acid to t...
Embodiment 2
[0029]Add 200 mL of anhydrous methanol and 16 g (0.70 mol) of sodium metal into a 2L four-neck flask, stir, and add 86.0 g (0.702 mol) of guanidine nitrate after it is completely dissolved, heat to 50°C, and stir for 0.5 hours. Add 56.0 g (0.486 mol) of ethyl cyanoacetate dropwise, heat to 50° C., react for 1 hour, and evaporate methanol to dryness under reduced pressure. Add 300 mL of formamide to dissolve, and cool to 4°C in ice water. Add 37g (0.535mol) of sodium nitrite, add dropwise a mixed solution of 100ml formamide and 80ml 98% formic acid, control the temperature not to exceed 10°C, and react at 10°C for 2 hours after dropping. Heat to 80°C, add 30.0 g (0.288 mol) of sodium bisulfite, and react at 80°C for 1 hour. Reaction was carried out at 150° C. for another 4 hours. Cool, filter and wash with water several times. After drying, 140.5 g of brown solid was obtained.
[0030] Add 1500 mL of 2N hydrochloric acid to the above solid, decolorize it with 10 g of activa...
Embodiment 3
[0032] Add 1000 mL of isopropanol and 76 g (1.95 mol) of potassium metal into a 2L four-neck flask, stir, and add 218.7 g (1.944 mol) of guanidine nitrate after it is completely dissolved, heat to reflux temperature, and stir for 2 hours. 112.0 g (0.972 mol) of ethyl cyanoacetate was added dropwise, heated to reflux temperature, reacted for 10 hours, and evaporated to dryness of isopropanol under reduced pressure. Add 1000mL of formamide to dissolve, cool to 4°C in ice water, add 100.5g (1.458mol) of sodium nitrite, add dropwise a mixed solution of 100ml of formamide and 110ml of 98% formic acid, and control the temperature below 5°C. After dropping, react at 4°C for 10 hours. Heat to 180°C, add 40.0 g (0.384 mol) of sodium bisulfite, and react at 180°C for 5 hours. 200°C for another 10 hours. Cool, filter and wash with water several times. After drying, 283 g of brown solid was obtained.
[0033] The above solid was recrystallized by adding 3000 mL of 3N hydrochloric acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com