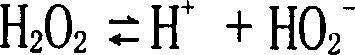Stabilizer for use in hydrogen peroxide degumming and bleaching of flax and its preparation method and application
A technology of flax hydrogen peroxide and stabilizer, which is applied to bleaching products, bast fiber produced by chemical method, textiles and papermaking, etc., can solve the problems of single use of stabilizer, poor effect, environmental pollution, etc., and achieve saving of chemical materials and funds, improve the operating environment, and avoid the effect of severe damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
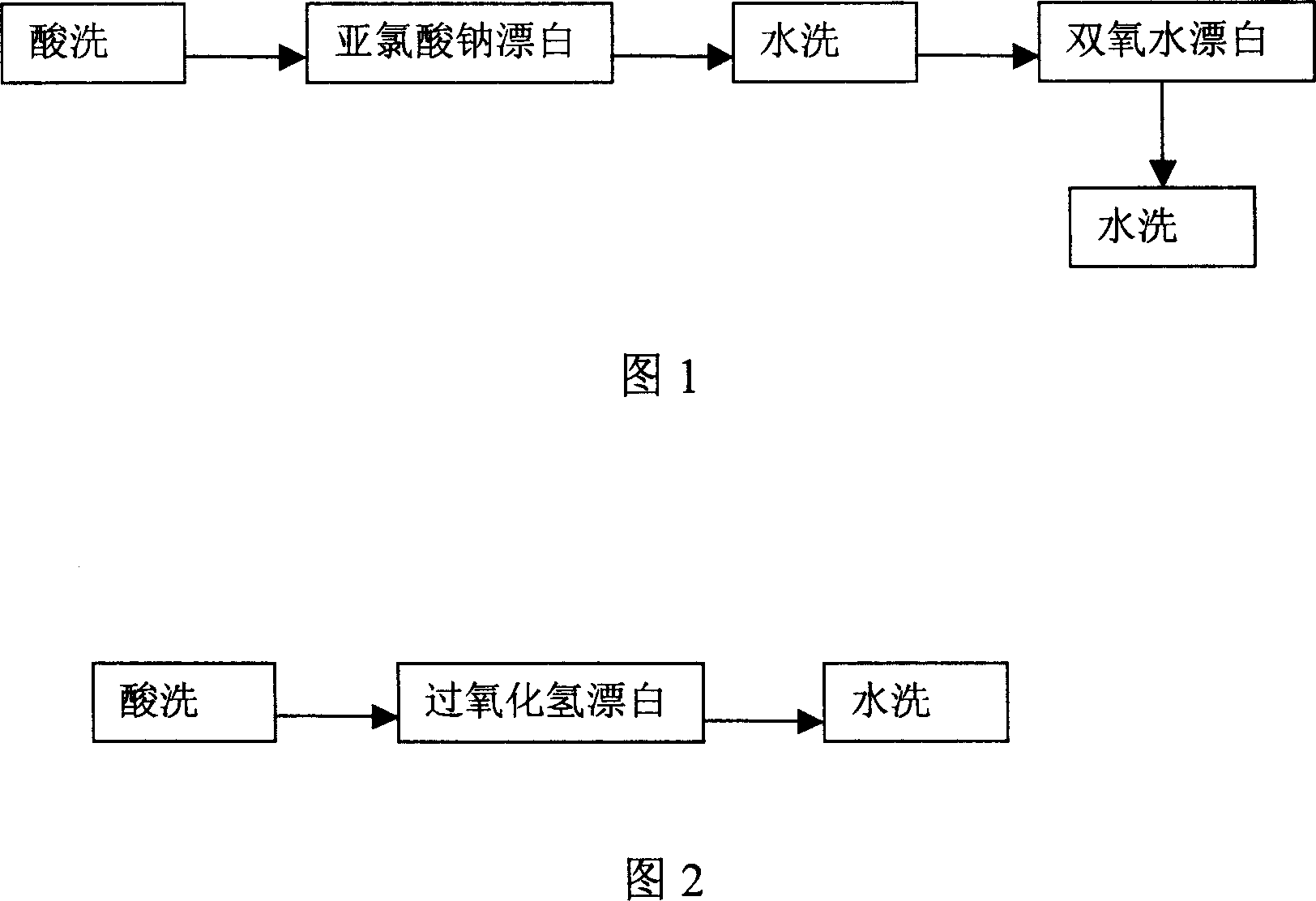
[0031] Please refer to accompanying drawing 2, the technological process of degumming and bleaching flax roving in this embodiment is shown in Figure 2.
[0032] The yarn to be degummed and bleached adopts flax roving (the roving made by Yulu hemp, and the roving count is 18Nm) 200g. Soak flax roving in sulfuric acid (sulfuric acid 15g / L, room temperature, 20 minutes) → wash with water, then use hydrogen peroxide solution (bath ratio 1:10, hydrogen peroxide consumption 6g / L, pH=10, penetrant (C 7-9 h 15-19 (CH 2 CH 2 O) 5 OH) 3g / L, stabilizer 8g / L, temperature 100°C) for 40 minutes, washed with water after treatment, and dried to complete the degumming and bleaching process of flax roving. Among them, the stabilizer (mass percentage) consists of 5% of diethylenetriaminepentamethylenephosphonic acid (DTPMP), 2% of sepiolite, 2% of montmorillonite, 1% of ferric oxide, 1% of magnesium oxide, oxidized 1% aluminum, 2% gluconic acid, 6% boric acid, 1% sodium silicate, 1% fatty ...
Embodiment 2
[0038] Please refer to accompanying drawing 2, the technological process of degumming and bleaching flax roving in this embodiment is shown in Figure 2.
[0039] The yarn to be degummed and bleached is 200g of flax roving (the roving spun by Yulu hemp, and the roving count is 20Nm). Soak flax roving in sulfuric acid (sulfuric acid 10g / L, room temperature, 30 minutes) → wash with water, then use hydrogen peroxide solution (bath ratio 1:15, hydrogen peroxide consumption 8g / L, pH=9.5, penetrant (C 7-9 h 15-19 (CH 2 CH 2 O) 5 OH) 5g / L, stabilizer 10g / L, temperature 100°C) for 60 minutes, washed with water after treatment, and dried to complete the degumming and bleaching process of flax roving. Among them, the stabilizer (mass percentage) consists of 8% of diethylenetriaminepentamethylenephosphonic acid (DTPMP), 4% of sepiolite, 6% of montmorillonite, 4% of ferric oxide, 4% of magnesium oxide, oxide Aluminum 3%, gluconic acid 6%, boric acid 10%, sodium silicate 2%, nonionic s...
Embodiment 3
[0046] Please refer to accompanying drawing 2, and present embodiment is shown in Figure 2 to the technological process of linen fabric bleaching.
[0047] The fabric to be bleached adopts linen fabric (plain weave, 220g / m 2 , 150×158 pieces / dm) 30×40cm piece. Squeeze the linen fabric with acid (sulfuric acid 20g / L, room temperature) → stack (room temperature, 20 minutes n) → wash with water, and then use hydrogen peroxide solution (hydrogen peroxide dosage 8g / L, pH=10.5, penetrant (C 7-9 h 15-19 (CH 2 CH 2 O) 5 OH) 3g / L, stabilizer 6g / L, temperature 100°C) for 40 minutes, washed with water after the treatment, and dried to complete the bleaching process of the linen fabric. Bleached linen was dyed using the following recipe. Among them, the stabilizer (mass percentage) consists of 10% of diethylenetriaminepentamethylenephosphonic acid (DTPMP), 3% of sepiolite, 6% of montmorillonite, 6% of ferric oxide, 4% of magnesium oxide, oxidized Aluminum 5%, gluconic acid 8%, bori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com