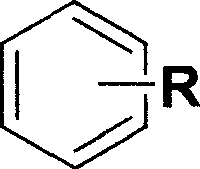
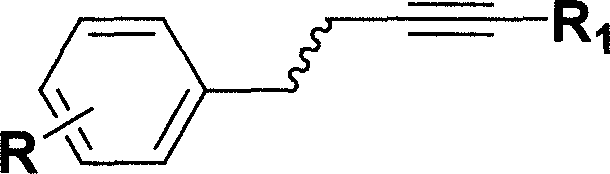
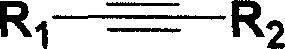Iron catalyzed styrene derivative synthesizing process with active arene and alkyne
A technology of styrene derivatives and compounds, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of trifluoroacetic acid increasing raw material costs, poor atom economy, increasing production costs, etc., to achieve Inexpensive, easy to handle, energy-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] At room temperature (20 °C), in a 10 mL round bottom flask was added trimethylbenzene (360.5 mg, 3.0 mmol), phenylacetylene (102.2 mg, 1.0 mmol) and FeCl 3 (16.2mg, 0.10mmol), stirred in 0.5mL nitromethane, after 5 hours, stopped the reaction, filtered, evaporated under reduced pressure to remove the solvent, separated by column chromatography to obtain the product 1-(2', 4', 6' -Trimethylphenyl)-1-styrene 142.3 mg, yield 64%.
Embodiment 2
[0027] At room temperature (20°C), trimethylbenzene (120.5 mg, 1.0 mmol), phenylacetylene (102.2 mg, 1.0 mmol) and FeCl were added to a 10 mL round bottom flask 3 (16.2 mg, 0.10 mmol), stirred in 0.5 mL of nitromethane. After 5 hours, the reaction stopped. The product 1-(2',4',6'-trimethylphenyl)-1-styrene was 142.3 mg as measured by gas chromatography, and the yield was 64%.
Embodiment 3
[0029] At room temperature (20°C), in a 10 mL round bottom flask was added trimethylbenzene (1200.5 mg, 10.0 mmol), phenylacetylene (102.2 mg, 1.0 mmol) and FeCl 3 (16.2 mg, 0.10 mmol), stirred in 0.5 mL of nitromethane. After 2 hours, the reaction stopped. The product 1-(2',4',6'-trimethylphenyl)-1-styrene was measured by gas chromatography to be 190.0mg, and the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com