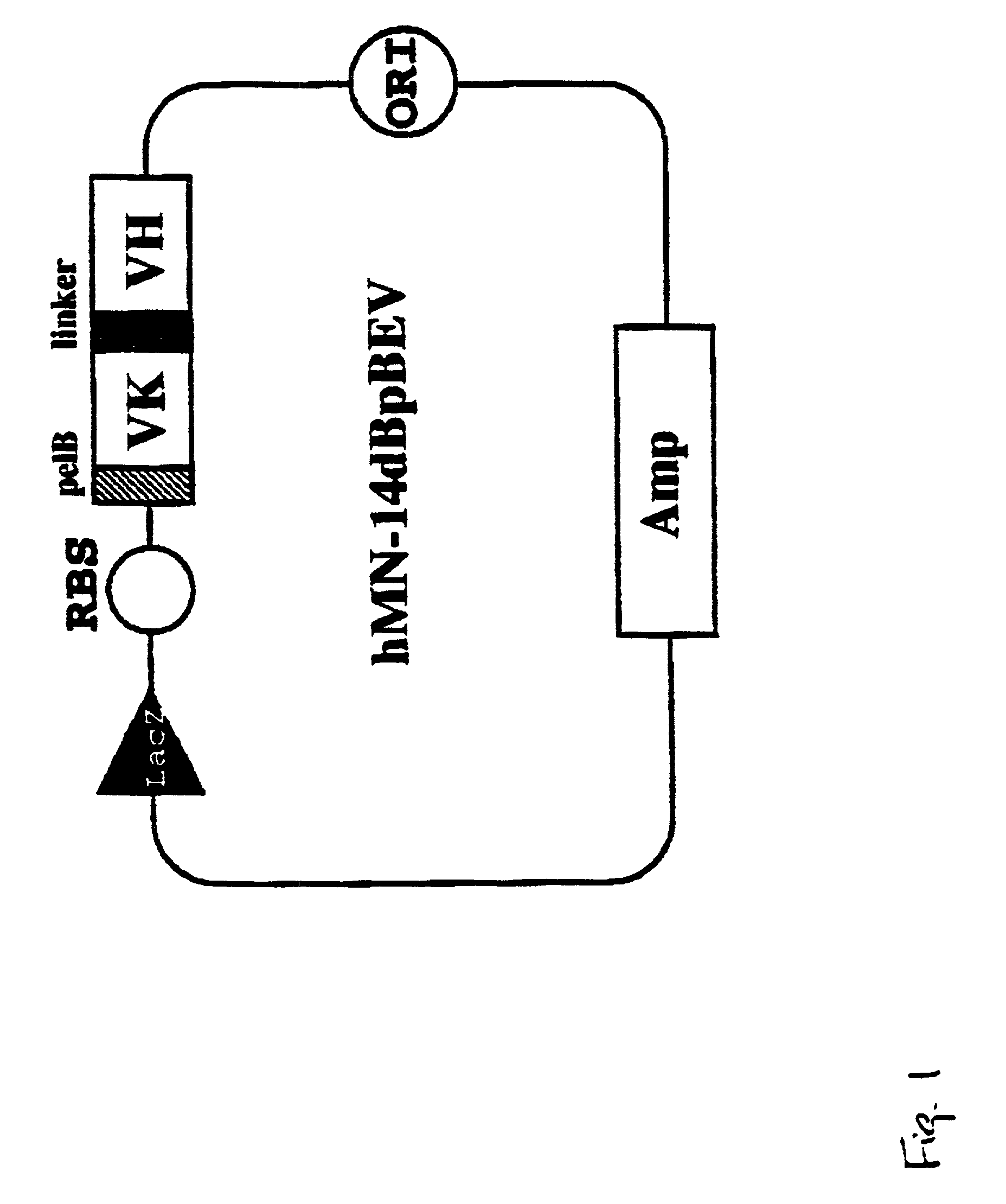
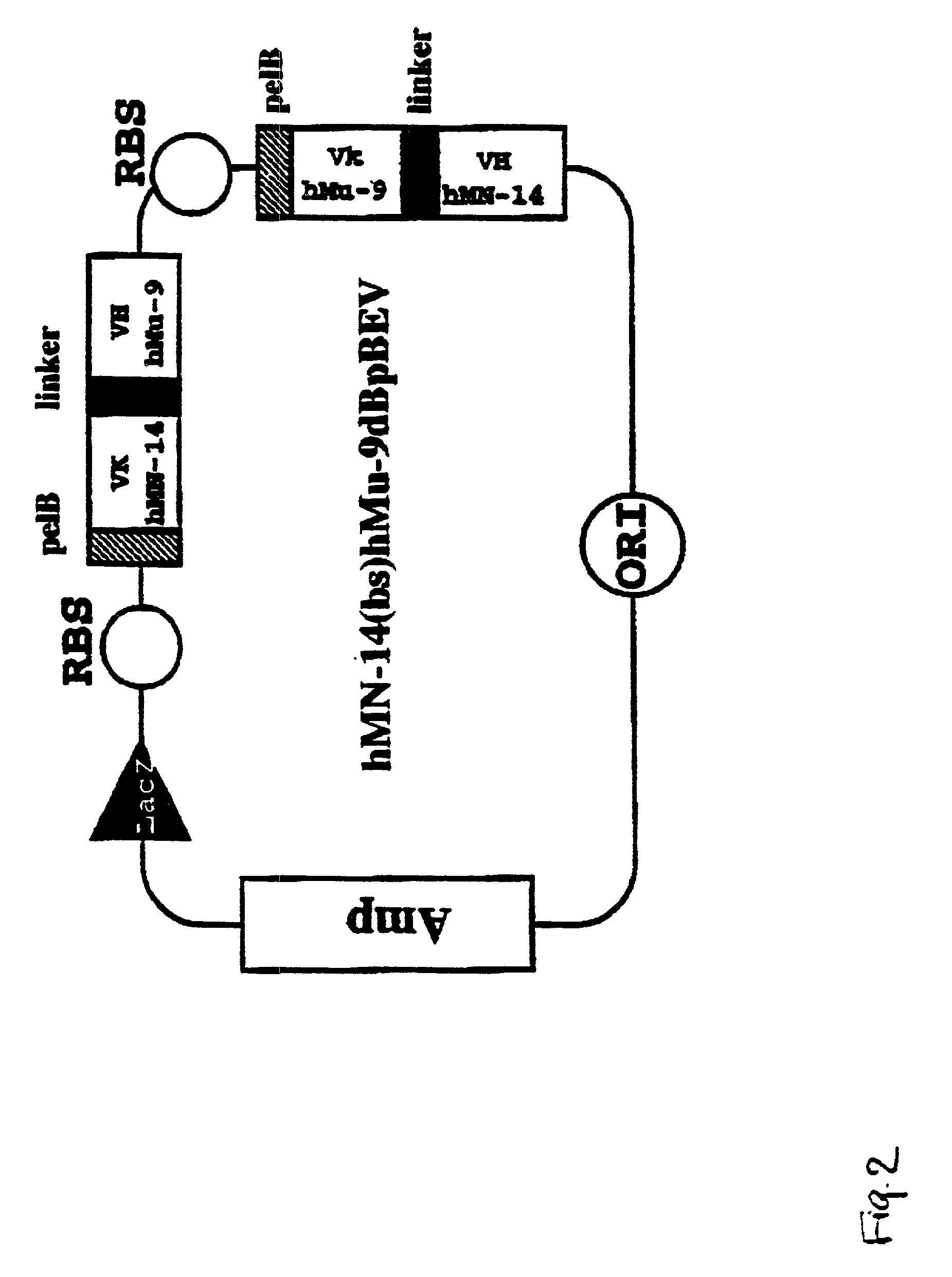
Intraoperative, intravascular and endoscopic tumor and lesion detection, biopsy and therapy
a tumor and lesion technology, applied in the field of intravascular and endoscopic tumor and lesion detection, can solve the problems of complicating diagnosis, inherently limited non-invasive raids, and becoming the limiting factor in their detection, and achieve the effect of enhancing the discrimination between tumors and non-tumor tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0127] Intraoperative Tumor Detection
[0128] A female patient with a cecal carcinoma recurrence is injected i.v. with Tc-99m-labeled murine monoclonal antibody NP-4 Fab' fragment against carcinoembryonic antigen (CEA) (15 mCi Tc-99m; 1 mg Fab'). The patient is scanned with a gamma camera, producing both planar and single-photon emission computed tomographic images at 3-5 hours later, and the cecal lesion is defined. Without delaying the planned surgery, the patient is brought to the operating room 8 hours after the antibody administration, and undergoes surgery for removal of the cecal tumor and any other sites of involvement in the region. This is achieved by passing a sterile radiation probe over the viscera to identify sites of tumor spread. The probe has a cadmium telluride scintillation crystal, collimator, preamplifier and amplifier, similar to that reported by Aitken et al., op. cit., but is collimated to select energy in the range of 100-160 keV. The counts registered are tra...
example 2
[0129] Endoscopic Tumor Detection
[0130] (A) A male patient with a suspected colonic polyp (having a history of recurrent polyps in the colon)is scheduled for a colonoscopy because of a recent positive guaiac test for hemoglobin in his stool, and an elevated blood CEA titer of 12.5 ng / ml. A dose of CEA monoclonal antibody NP-4 F(ab').sub.2 labeled with I-125 by the chloramine-T method (2 mg F(ab').sub.2with 1.0 mCi I-125) is injected i.v. and 24 hours later, without delaying the planned endoscopic procedure, the patient undergoes endoscopy, using a colonoscope equipped with a radiation detector capable of measuring the radiation emitted by I-125. The detector comprises a cadmium telluride scintillation crystal mounted on the tip of an optical fiber waveguide. The optical fiber is housed within a shielded tube which itself is inserted inside the colonoscope and extends to within about 4 mm of the open end thereof, the remaining length of tubing serving as a collimator. The other end o...
example 3
[0132] Intraoperative Tumor Therapy
[0133] A woman with ovarian cancer having extensive abdominal spread is injected prior to surgery with a biotinylated preparation of RS7-3G11 monoclonal antibody (3 mg) i.v. Three days later a chase dose of avidin (10 mg) is given i.v. in 2 divided doses 60 minutes apart. Twenty-four hours later, a 1.5 mg dose of biotinylated RS7-3G11 conjugated with DTPA-indium (stable) is injected i.v. The next day, the patient undergoes a resection of all visible and palpable tumors in her abdominal cavity, followed by intraoperative irradiation of the exposed cavity with monochromatic X-rays of 40 keV to destroy micrometastatic cancer spread. At 6 and 9 months later, no evidence of disease is present, and the patient's blood CA-125titer is within the normal range, as contrasted to its marked elevation prior to treatment
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com