Use of pro-apoptotic factors in treatment of atherosclerosis
a pro-apoptotic factor and atherosclerosis technology, applied in the field of vascular disease, can solve the problems of reduced lumen size and tissue perfusion, limited blood flow through the vessel, and treatment does not achieve an acute reduction in the size of the vascular lesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A20 Activity in SMC
[0121] A20 is Part of the Physiological response of SMC to TNF
[0122] Primary human aortic SMC were cultured in 6 well plates using smooth muscle basal medium (SmBM) (Clonetics, California) supplemented with growth factors, Gentamycin and Amphotericin B. Confluent cells were stimulated with 100 Units of recombinant human (rhu)TNF and RNA was extracted before and at 1 and 6 hours (h) following addition of TNE. RNA samples were analyzed by Northern blot analysis for the expression of A20 using an A20 cDNA probe (Ferran et al., supra). In all experiments, a cDNA probe for human GAPDH was used to evaluate equal loading of RNA. Results showed almost no A20 mRNA expression prior to TNF addition. A20 mRNA was strongly induced 1 h following TNF stimulation and started declining 6 h thereafter (FIG. 1A). The induction of A20 by TNF was confirmed at the protein level by means of immunohistochemistry using a rabbit anti-human A20 polyclonal antibody developed in the laborator...
example 2
Evaluation of the Effects of Expressing A20 in SMC In Vitro on Inhibition of NF-.kappa.B Activation and the Impact on SMC Activation and Proliferation Induction of A20
[0143] One may determine whether A20 expression is part of the physiological response of the SMC to injury.
[0144] Fourth to sixth passage human aortic SMC monolayer cultures are activated using different agonists that are relevant to the pathogenesis of atherosclerosis. These agonists include TNF, LDL, and oxidized LDL, growth factors (platelet derived growth factor (PDGF)) and CD40 cross-linking. The expression of A20 in SMC following these agonists is evaluated at different time points (1-24 h), both at the mRNA and protein levels as described in the preliminary results. One then tests whether each of these critical mediators of the atherosclerotic lesion will, like TNF, induce the expression of A20. We propose, without limiting the biochemical mechanism of the invention, that A20 induction is part of the "regulatory...
example 3
A20 Activity In Vivo and In Vitro
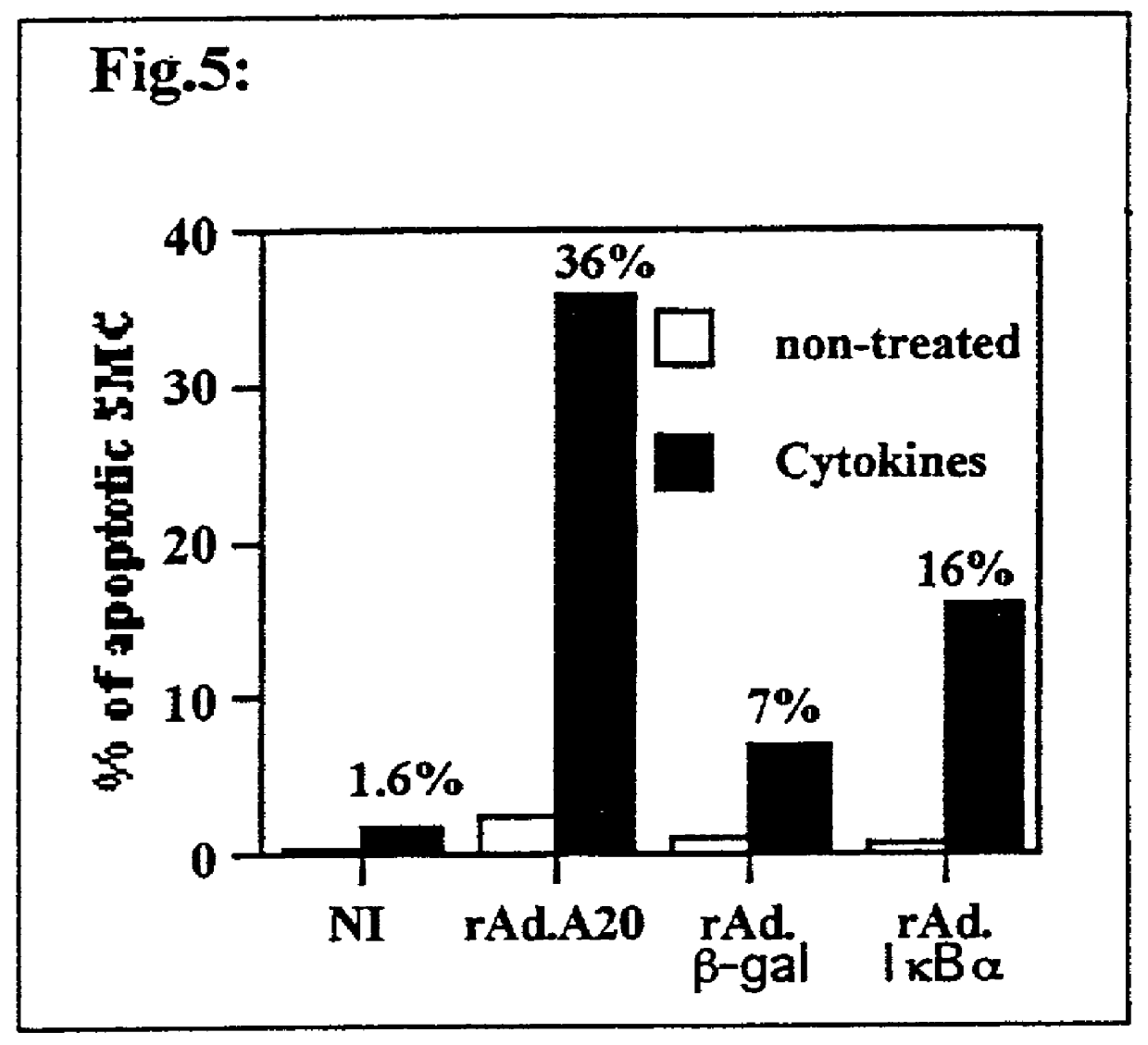
[0154] Impact of A20 Overexpression Upon SMC Apoptosis In Vitro
[0155] Apoptosis is now viewed as beneficial to prevent redevelopment and promote the regression of established atherosclerotic lesions. The present invention demonstrates for the first time that A20 sensitizes SMC to cytokine-mediated apoptosis, making A20 a prime gene therapy target to achieve this aim. These experiments are planned to extend our finding that A20 sensitizes SMC to cytokine-mediated apoptosis and to other apoptotic stimuli that are present within the atherosclerotic plaque i.e. Fas, NO and oxidized LDL. Second, one may determine the molecular basis of the effect of A20 upon the death signaling machinery. These studies evaluate the effect of A20 on activation of caspases, mitochondrial membrane potential, c-myc, cytochrome c release and cleavage of death substrates such as PARP that are the hallmarks of apoptosis.
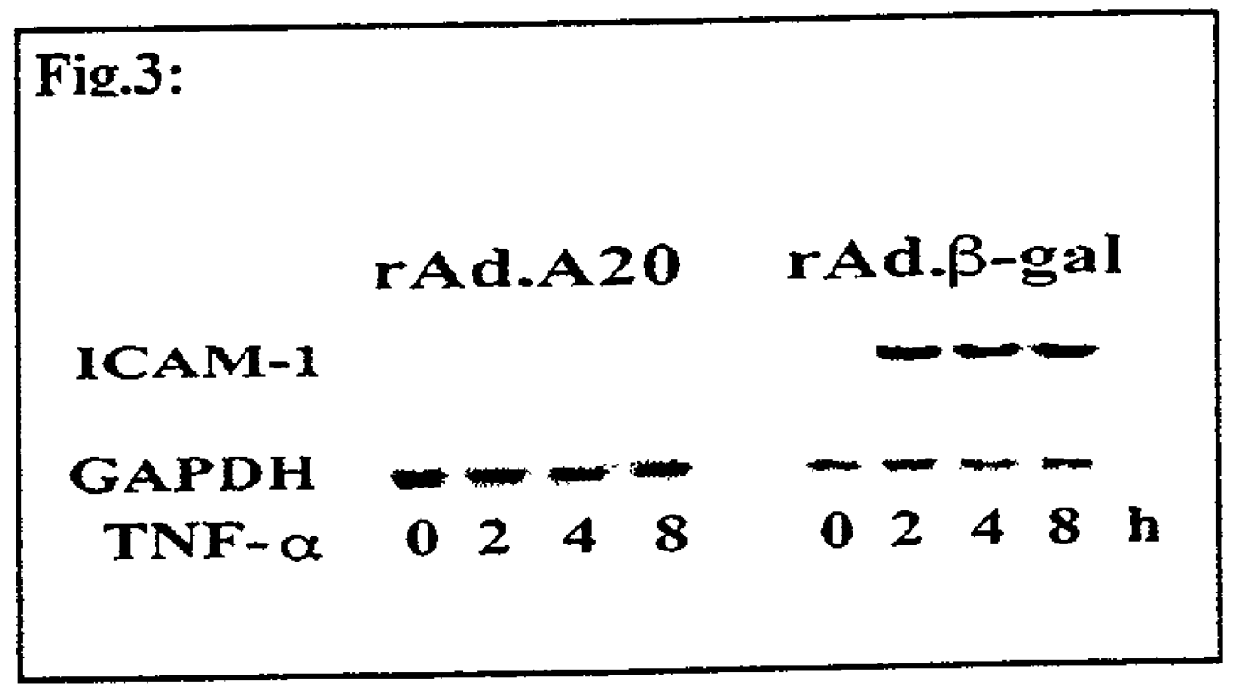
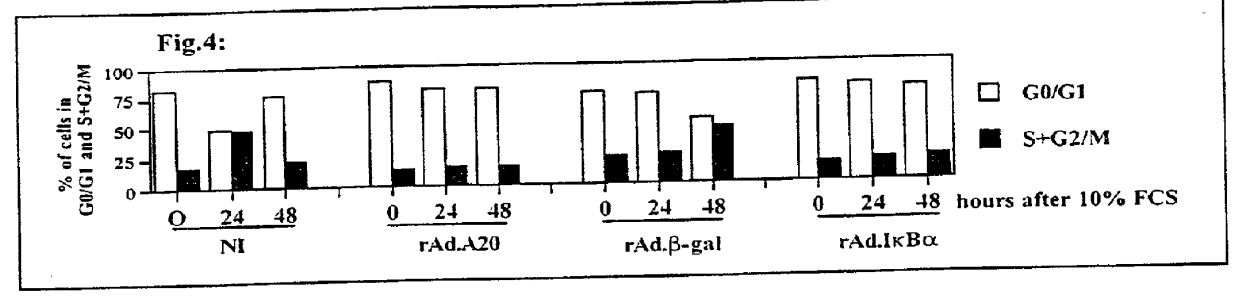
[0156] As described above, non-infected SMC and SMC infecte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com