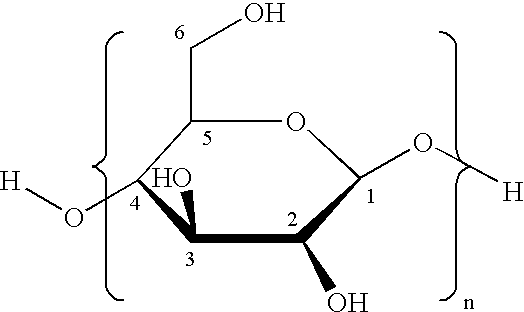
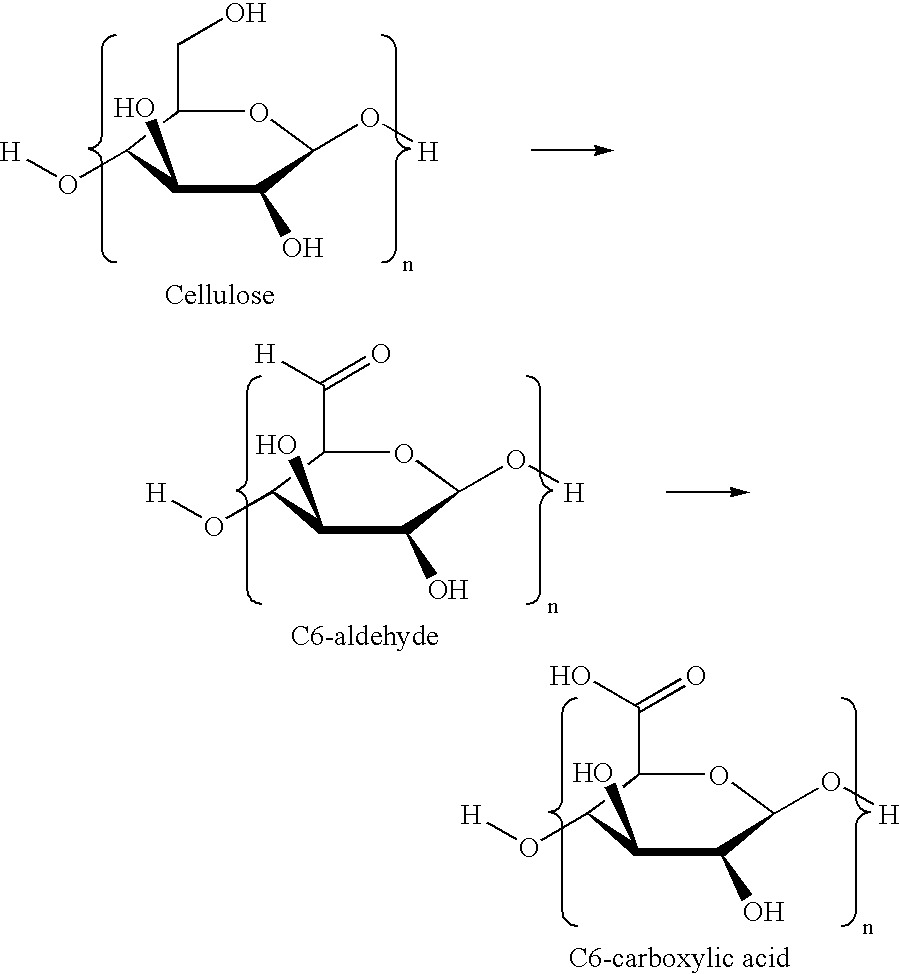
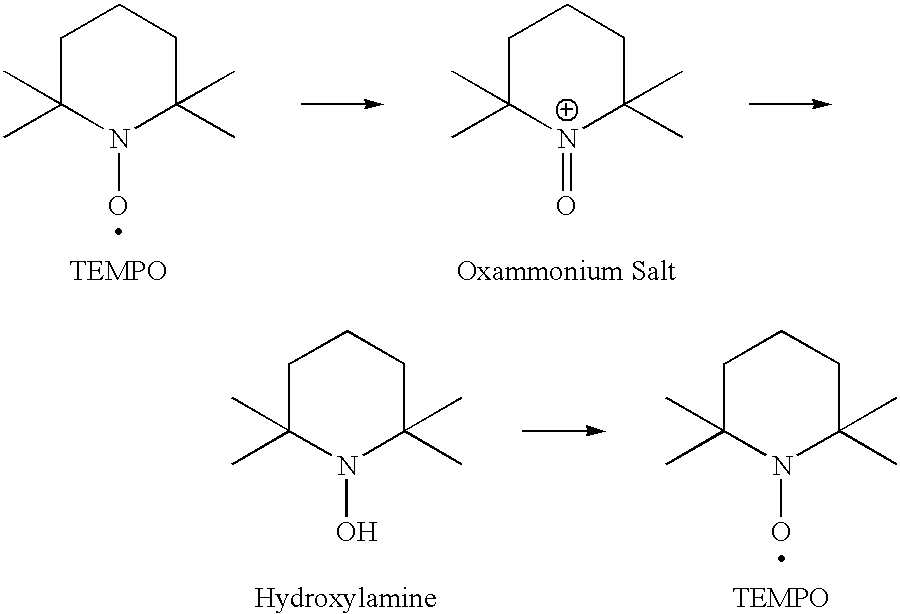
Method for preparation of stabilized carboxylated cellulose
a technology cellulose, which is applied in the field of preparation of stabilized carboxylated cellulose, can solve the problems of poor reproduction of the tempo system, inability to obtain water soluble materials, and equivocality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Effect on Cellulose D.P. after Caro's Acid Oxidation
[0089] An oxidized cellulose sample was prepared in similar manner to that of Example 1 except that the pulp used was a never dried sample of northern mixed conifer bleached kraft furnish obtained from a Weyerhaeuser Company Grand Prairie, Alberta mill. The Caro's acid was prepared from K.sub.2S.sub.2O.sub.8 and 98% sulfuric acid and diluted with deionized water to give 60 mL of a 0.28% solution. This was further diluted with 60 mL of deionized water and adjusted to pH 7.5 with NaHCO.sub.3. The oxidation catalyst was prepared by dissolving 0.012 g of the 1,3-propanediol ketal of 2,2,6,6-tetramethyl-4-piperidone-1-oxyl in the Caro's acid solution. Then 51 g (12.5 g O.D.) was suspended in the basic Caro's acid solution and finally 0.25 g of NaBr was added and mixed well. The mixture was placed in a polyethylene bag and heated in a water bath for 15 minutes at 60.degree. C. The fiber was filtered off and washed well in deionized water...
example 3
Stabilization of Oxidized Cellulose using Caro's Acid Under Acidic Conditions
[0092] An additional sample of the never dried Alberta pulp was oxidized using 7,7,9,9-tetramethyl-1,4-dioxa-8-azaspiro[4.5]decane-2-methanol rather than TEMPO. This material is also designated as the glyceryl ketal of triacetoneamine. A 145 mg portion of the amine was dissolved in 250 g of 0.28% basic Caro's acid solution at pH 7.5. A slurry of 102 g never dried Grand Prairie kraft pulp (25 g O.D.) was then dispersed in the solution. The mixture was placed in a plastic bag and 500 mg NaBr was added and dispersed throughout the mixture. The bag was sealed and placed in a water bath at 60.degree. C. for 30 minutes. The oxidized cellulose was drained and thoroughly washed with deionized water. A small portion was retained for analysis and the remainder divided into two parts.
[0093] A first 30 g portion of the oxidized cellulose (8.0 g O.D.) was dispersed in 500 mL of Na.sub.2HPO.sub.4 / citric acid buffer solut...
example 4
1,3-Propanediol Ketal of Triacetone Amine Used to Generate its Oxammonium Salt In Situ
[0096] A Caro's acid stock solution was prepared using 200 g of 98% H.sub.2SO.sub.4 and 40 g of 70% H.sub.2O.sub.2. An 0.80 g portion of this was added to 100 g of deionized water and the pH raised to 7.5 with Na.sub.2CO.sub.3. The concentration of Caro's acid was 0.28% and of H.sub.2O.sub.2 0.02% by weight. Into this solution was dispersed 51 g (12.5 g O.D.) of the never dried Alberta pulp of Example 2. A catalyst solution was made by dissolving 0.0048 g of the 1,3-propanediol ketal of triacetoneamine and 0.250 g of NaBr in 50 g of a solution brought to pH 7.5 with NaHCO.sub.3. This was added to the cellulose slurry in Caro's acid solution and the mixture was placed in a polyethylene bag and immersed in a 60.degree. C. water bath for 15 minutes. After the initial oxidation the pulp was drained and washed and a small sample taken for analysis.
[0097] The oxidized cellulose was then dispersed in 500 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com