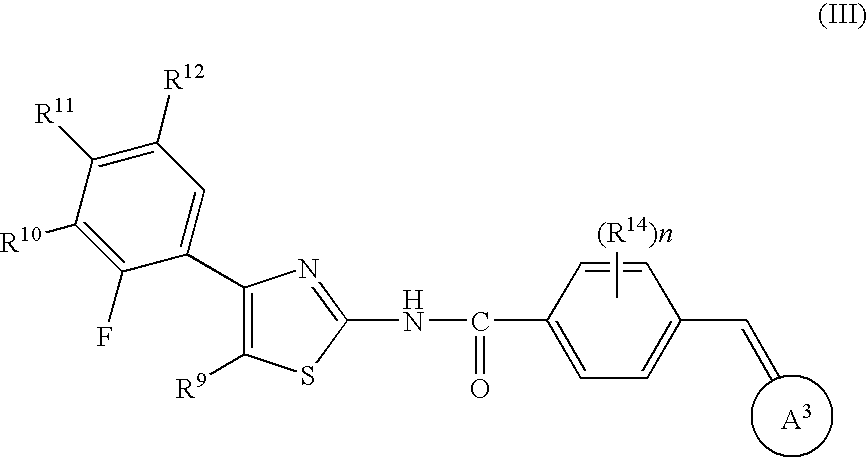
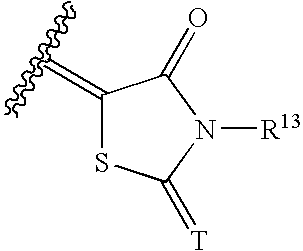
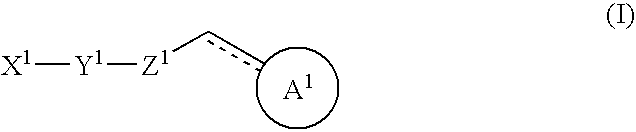
Halogen compounds having thrombopoietin receptor agonism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0186] Preparation of Compound (A-1) 20
[0187] (Step 1)
[0188] To a solution of 2'-fluoro-3'-trifluoroacetophenone (1) (20.9 g) in 10% methanol-chloroform was added bromine (5.26 mL), and the reaction mixture was stirred at room temperature until a color of bromine was disappeared. The solvent was evaporated under reduced pressure and the residue was dissolved in ethanol again. To the reaction mixture was added thiourea (7.71 g) and the mixture was heated under reflux for 2 h. To the reaction mixture was added a mixture of ethyl acetate-saturated aqueous sodium bicarbonate solution, and the organic layer was dried over anhydrous magnesium sulfate, and evaporated. The residue was purified by column chromatography to give a compound (2) (19.8 g).
[0189] .sup.1H NMR (CDCl.sub.3, .delta. ppm): 8.22-8.28 (m, 1H), 7.48-7.54 (m, 1H), 7.24-7.30 (m, 1H), 7.10 (d, 1H, J=2.4 Hz), 5.14 (bs, 2H).
[0190] (Step 2)
[0191] To a solution of methyl terephthalaldehydate (25 g) and rhodanine (23.3 g) in tolu...
formulation example
Formulation Example 1
[0211] Granules are prepared using the following ingredients.
8 Ingredients The compound represented by the formula (I) 10 mg Lactose 700 mg Corn starch 274 mg HPC-L 16 mg 1000 mg
[0212] The compound represented by the formula (I) and lactose are made pass through a 60 mesh sieve. Corn starch is made pass through a 120 mesh sieve. They are mixed by a twin shell blender. An aqueous solution of HPC-L (low mucosity hydroxypropylcellulose) is added to the mixture and the resulting mixture is kneaded, granulated (by the extrusion with pore size 0.5 to 1 mm mesh), and dried. The dried granules thus obtained are sieved by a swing sieve (12 / 60 mesh) to yield the granules.
Formulation 2
[0213] Powders for filling capsules are prepared using the following ingredients.
9 Ingredients The compound represented by the formula (I) 10 mg Lactose 79 mg Corn starch 10 mg Magnesium stearate 1 mg 100 mg
[0214] The compound represented by the formula (I) and lactose are made pass through a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Electrical inductance | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com